Testing hydrogen
Introduction Hydrogen is a flammable gas. It burns in air to produce water. A convenient test for hydrogen is to put a lighted splint in the mouth of a test tube full of the gas . The gas will burn with a characteristic squeaky pop if hydrogen is present. Hydrogen gas can be used as a fuel. When hydrogen burns in air it combines with ox...
Transition elements
OCREdexcelAQA Transition elements : properties Ligands and complex ions Ligand substitution and precipitation Redox reactions Electron configurations The video here uses the relevant section of the Royal Society of Chemistry's periodic table website to show the electron configurations of the first row of the transition ...
1.18 - 1.24 The periodic table
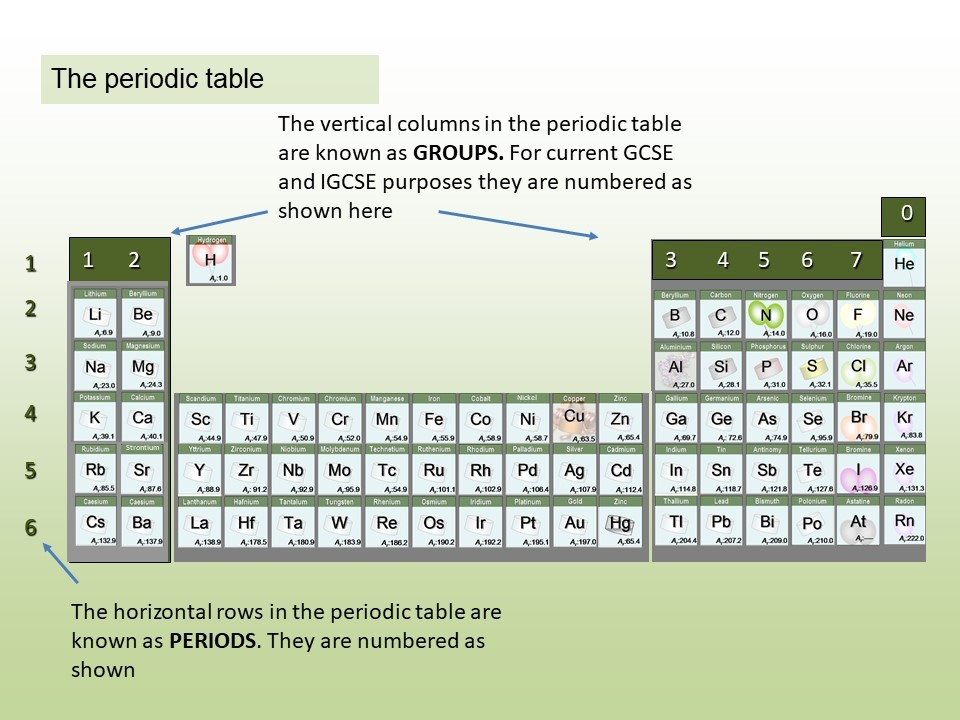
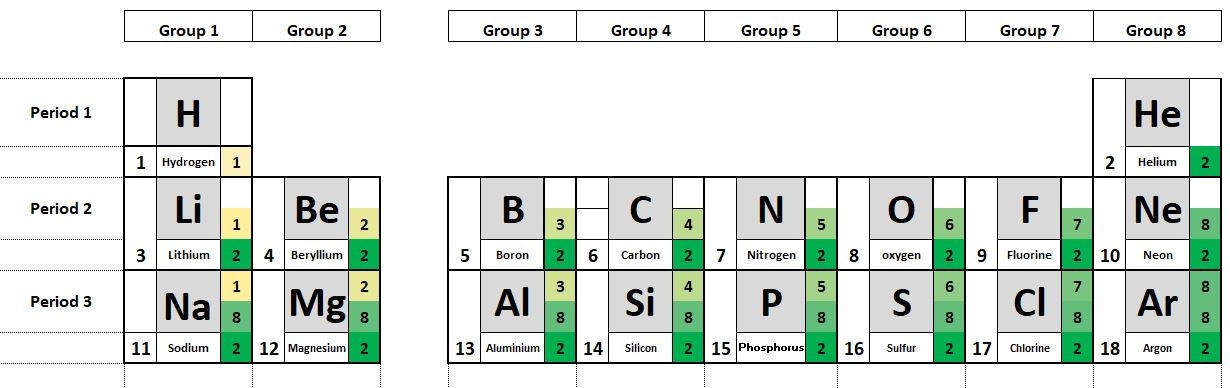
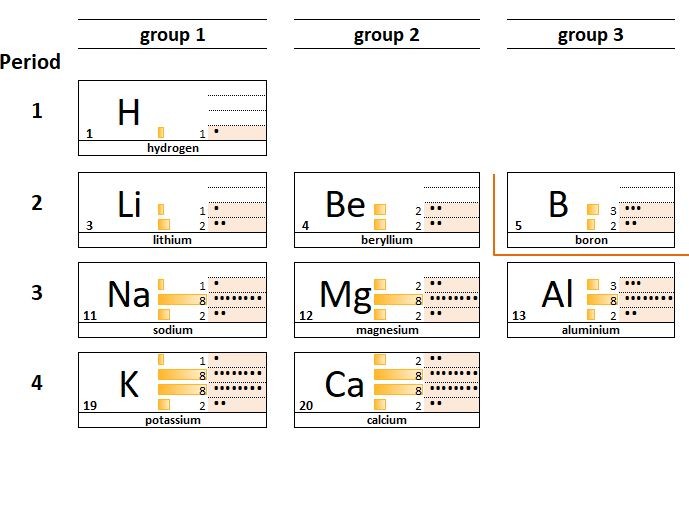
The elements in the periodic table are organised in order of increasing atomic number.
1.18 Organising the elements
The elements in the periodic table are organised in order of increasing atomic number.
1.19 Electron configurations
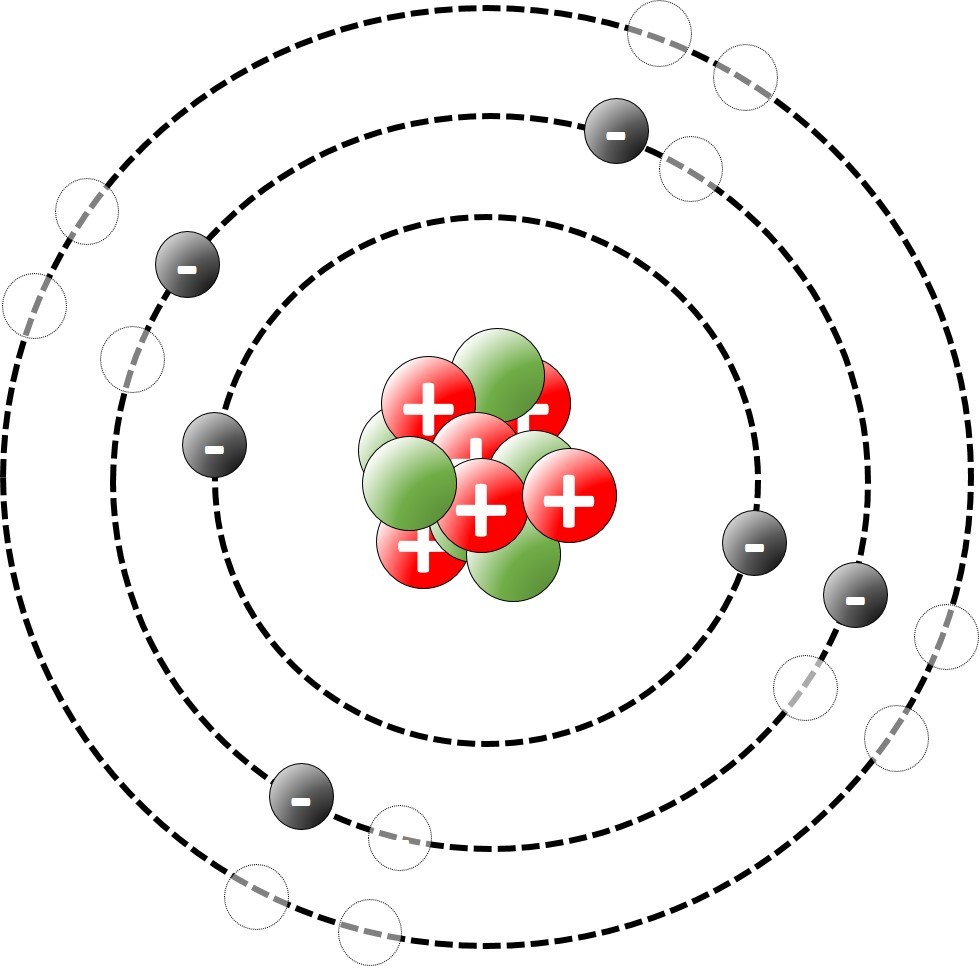
1.19 Deducing electron configurations Students should: 1.19 understand how to deduce the electronic configurations of the first 20 elements fromtheir positions in the Periodic Table A carbon atom has 6 protons and therefore 6 electrons. The electrons are arranged in two shells; 2 electrons in the first shell and 4 electrons in...