1.10 Crystallisation
Crystallisation can be used to separate a solid solute from its solvent. Re-crystallisation is used to purify samples obtained in the laboratory. But what is a crystal?
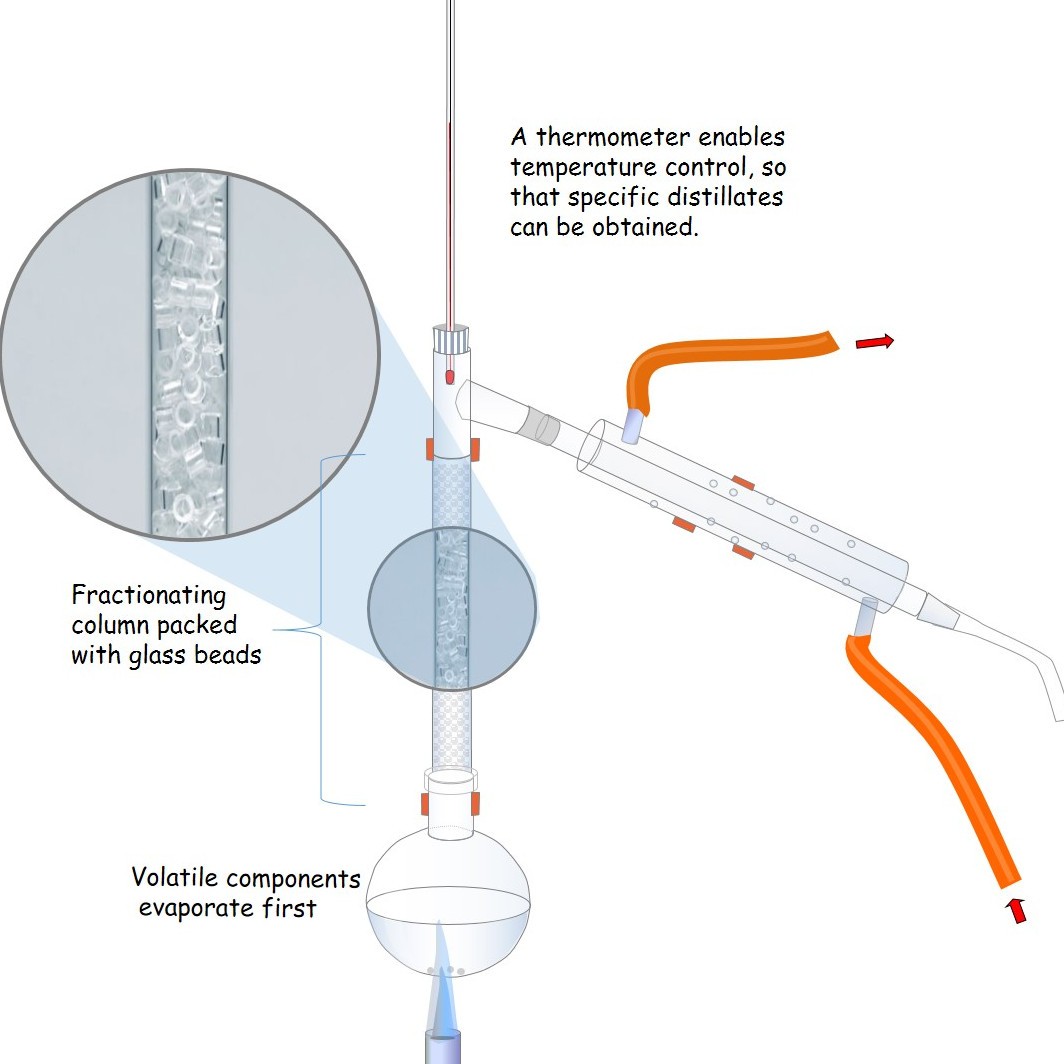
1.10 Fractional distillation
Find out about fractional distillation ; why it is different from simple distillation and how it can be used industrially
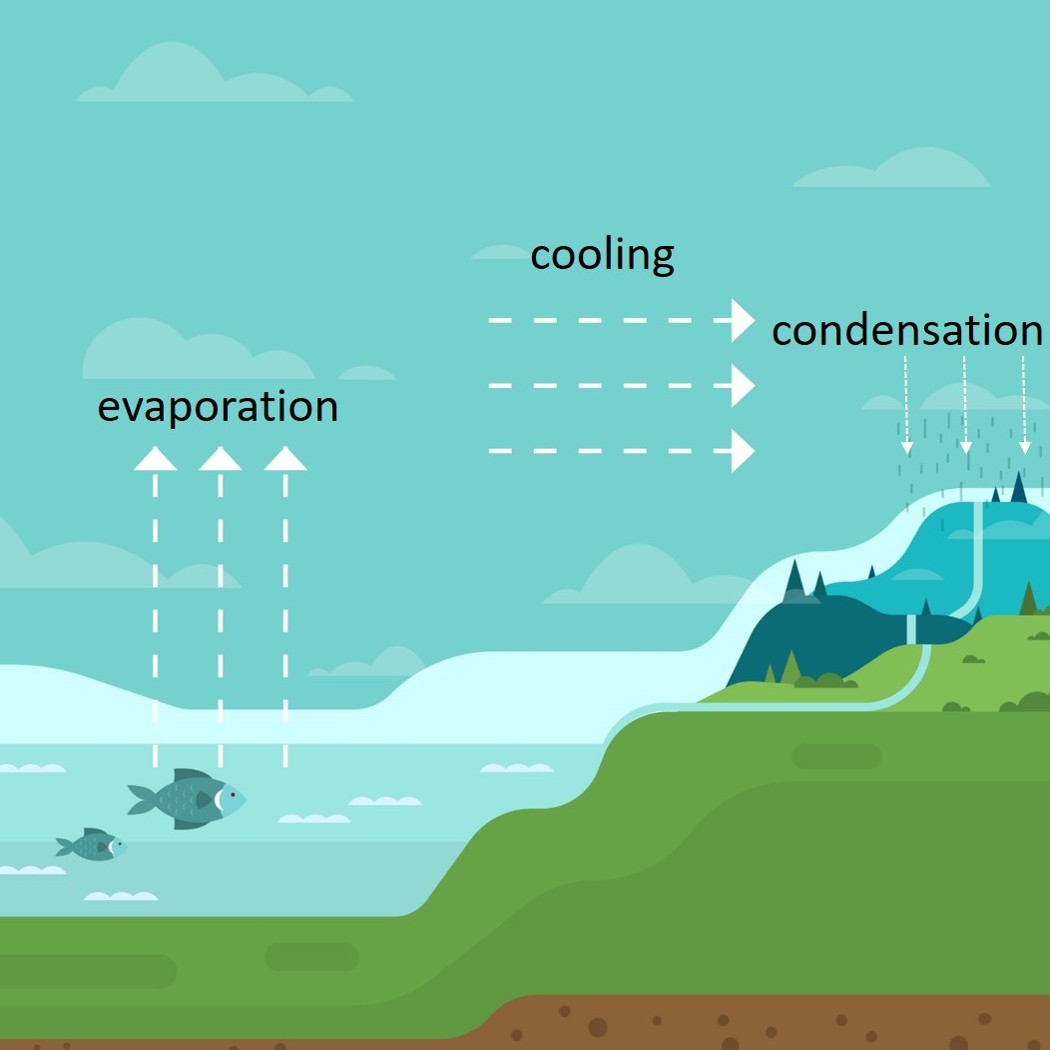
1.10 Simple distillation
Distillation is used to separate liquids from one another. Visit this page to see distillation in the laboratory and look at an industrial scale process.
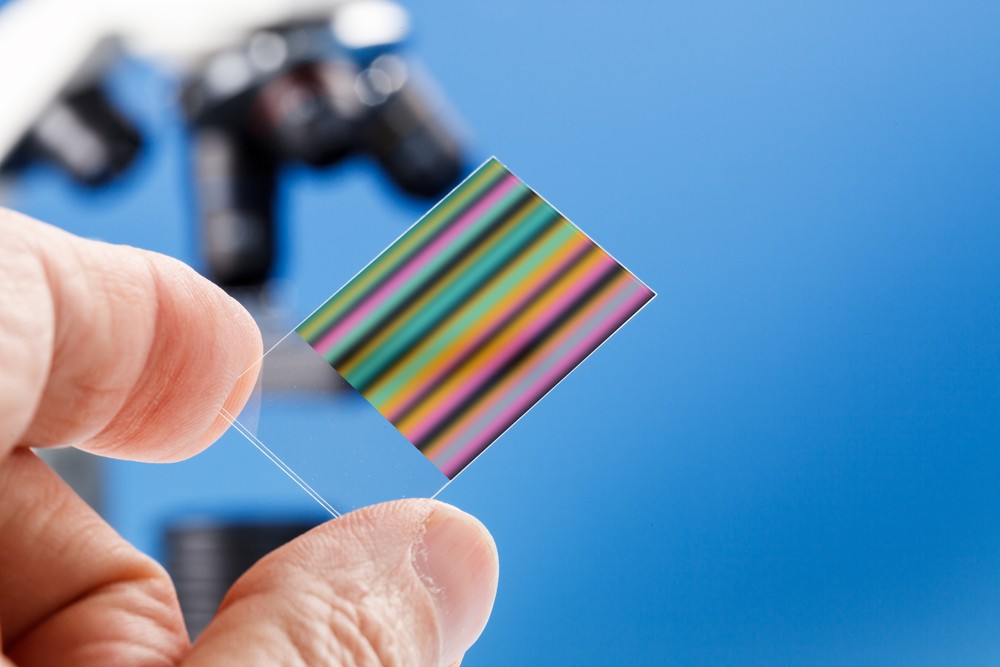
1.10 - 1.13 Chromatography
Chromatography is another important separation technique. Several different types of chromatography exist.
1.10 Filtration
Filtration can be a simple technique used to separate insoluble solids from a mixture or suspension. It has a lot of everyday applications.
compounds, elements or mixtures ?
Sun and air? Study the seaside image for a few seconds . Try to describe it: You might say that the image shows : A calm sea, clear blue sky, bright sunshine and a perfectly smooth beach. You could describe it in terms of the states of matter visible: SOLID Sand LIQUID Sea GASAirGAS and PLASMASun But as a chemist you might be as...
compounds, elements or mixtures, ecosystems or environments?
Sun and air? Study the seaside image for a few seconds . Try to describe it: You might say that the image shows : A calm sea, clear blue sky, bright sunshine and a perfectly smooth beach. You could describe it in terms of the states of matter visible: SOLID Sand LIQUID Sea GASAirGAS and PLASMASun As a chemistry student you might be...
Sun and air Study the seaside image for a few seconds . Try to describe it: You might say that the image shows : A calm sea, clear blue sky, bright sunshine and a perfectly smooth beach. You could describe it in terms of the states of matter visible: SOLID Sand LIQUID Sea GASAir GAS + PLASMASun But as a chemist y...
The Dead Sea is below sea level, this means that it does not drain so that although the water evaporates, the salt will stay, this means that the dead sea is a saturated solution. You can see this because salt crystals appear around the shore line. The oceans become salty for the same reason - soluble salts are washed in to the oceans by rive...
Crude oil is a major source of raw material for the petrochemical industry.
A question of balance
Many chemical reactions can be reversed. When this happens we can describe a forward reaction and a back reaction. Sometimes the forward and back reaction take place at equal rates. When this happens an Equilibrium is established Students should: 3.17 know that some reactions are reversible and this is indicated by the symbo...
Students should: 1.3 understand how the results of experiments involving the dilution of coloured solutionsand diffusion of gases can be explained This classic demonstration is used to demonstrate diffusion rates QuestionsAnswers in this reaction the two reactants are ammonia and hydrogen chloride write down the formula of each of these compo...
An exothermic reaction
3.1 Energetics Students should: 3.1 know that chemical reactions in which heat energy is given out are described asexothermic, and those in which heat energy is taken in are described as endothermic Every chemical reaction has an accompanying change in energy. Some reactions release energy to the surroundings and are known as Exothermic...
Universal indicator solution can be used to indicate the acidity or alkalin
Enter your text here ... IntroductionPearson specification A lot of everyday substances including some foods contain Acids. You can find alkalis amongst a number of common cleaning products. Acids are neutralised by alkalis to form salts and water. Here we consider the ways in which these reactions can be used...