1.47 - 1.48 Simple molecular substances
Look at the images of ice and diamond. In some senses you might say the two substances are similar. Both substances are solid, transparent and crystalline. In terms of bonding, they have similarities too : In ice, atoms of oxygen are held to hydrogen atoms by covalent bonds. In diamond atoms of carbon ar...
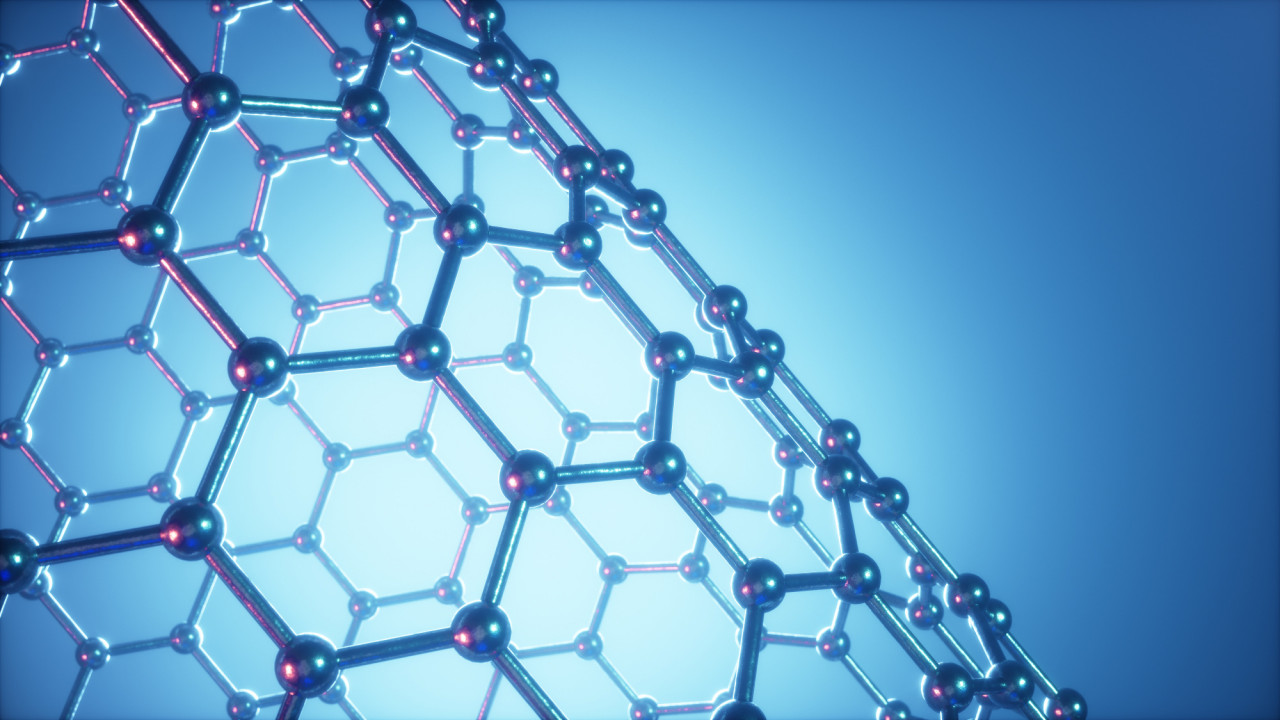
1.49 - 1.51 Covalent Giants
Covalent bonds form when electrons are shared between nuclei Atoms which can form two or more covalent bonds can bond with other similar atoms or themselves and form covalent lattices. Carbon in the form of diamond is a good example, as is silicon dioxide in the form of quartz. A valuable lattice Activity. Diamond - clos...
Food for thought
Intro image Why there might still be hope.. Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ...
Aromatic compounds
Intro image OCROCR 2EdexcelAQA Enter your text here ... Enter your text here ... I had a dream! This presentation outlines the way in which Kekule came to propose his model for the structure of benzene. It also outlines the ways in which the kekule proposal does not adequately explain all the properties of the benzene molecu...
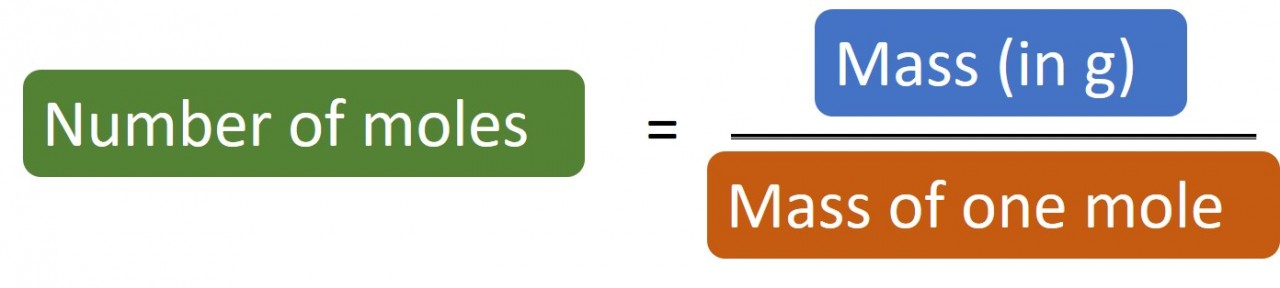
1.31 Finding formulae
Finding the formula of a compound is important to chemists. We can often predict what the formula might be because we know something about the electron configurations and the way atoms reorganise their electrons to achieve full or empty outer shells. However, here we look at a number of reactions where accurate mass measurement...