If a nucleus was the size of a raisin, the rest of the atom would be the size of a sports stadium
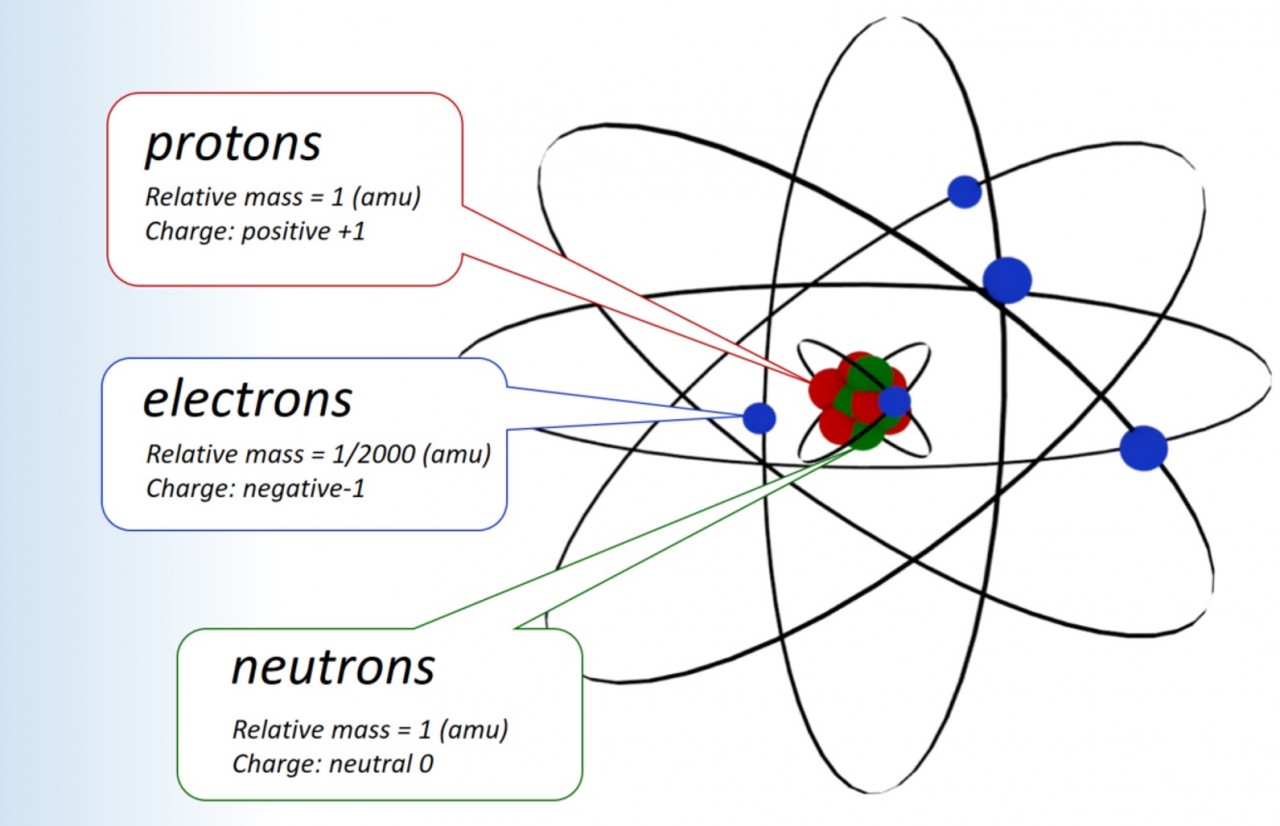
1.15 Sub atomic particles Students should: 1.15 know the structure of an atom in terms of the positions, relative masses and relativecharges of sub-atomic particles. Here we look at the particles inside the atom. Sub atomic particles are the particles found inside atoms. These include protons, neutrons and electrons: protons a...
1.55 Using electrolysis Electrolysis happens when an electric current is passed through an ionic compound which has been melted or dissolved. Electrolysis has a wide range of uses including : electroplating, extraction of aluminium, production of chlorine and the purification of copper . This post helps you to unders...
1.3 Activity 4. A particular problem Students should: 1.3 understand how the results of experiments involving the dilution of coloured solutions and diffusion of gases can be explained. Particle theory states that all matter consists of many, very small particles which are constantly moving or in a continual state of motion. T...
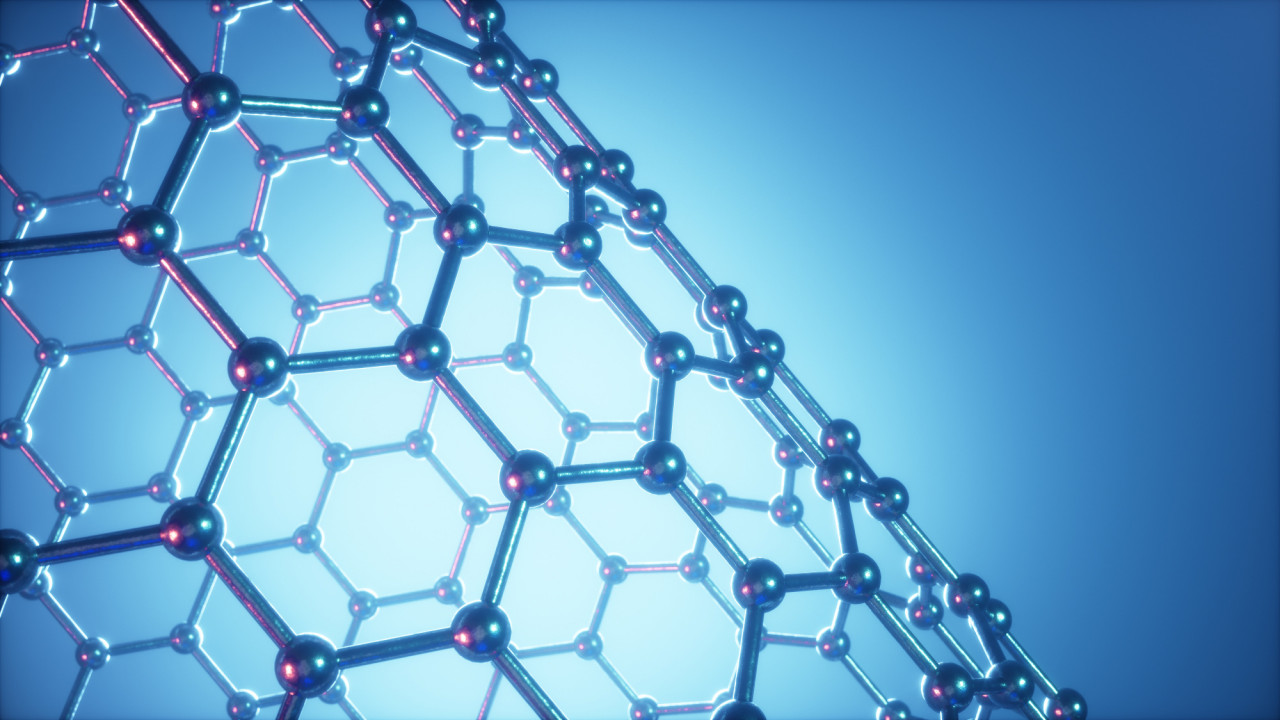
Covalent bonds form when electrons are shared between nuclei Atoms which can form two or more covalent bonds can bond with other similar atoms or themselves and form covalent lattices. Carbon in the form of diamond is a good example, as is silicon dioxide in the form of quartz. A valuable lattice Activity. Diamond - clos...
Look at the images of ice and diamond. In some senses you might say the two substances are similar. Both substances are solid, transparent and crystalline. In terms of bonding, they have similarities too : In ice, atoms of oxygen are held to hydrogen atoms by covalent bonds. In diamond atoms of carbon ar...
Look at the images of ice and diamond. In some senses you might say the two substances are similar. Both substances are solid, transparent and crystalline. In terms of bonding, they have similarities too : In ice, atoms of oxygen are held to hydrogen atoms by covalent bonds. In diamond atoms of carbon ar...
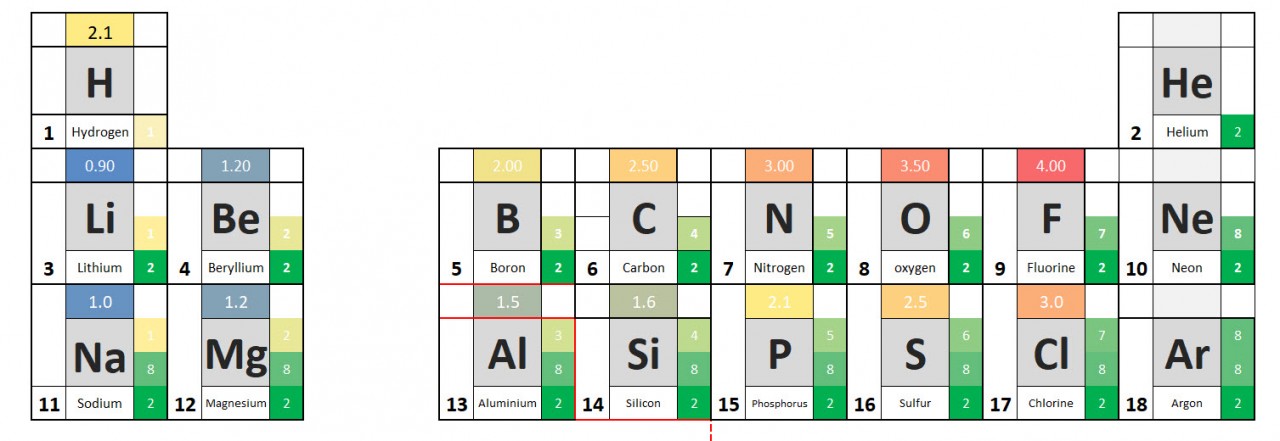
1.38 Activity. Know your ions. Students should: 1.38 know the charges of common ions listed You will have noted from the video that metal atoms tend to lose the electrons in their outermost electrons and form positive ions. The charge of the ion formed is equal to the number of electrons lost. Non metal atoms gain el...
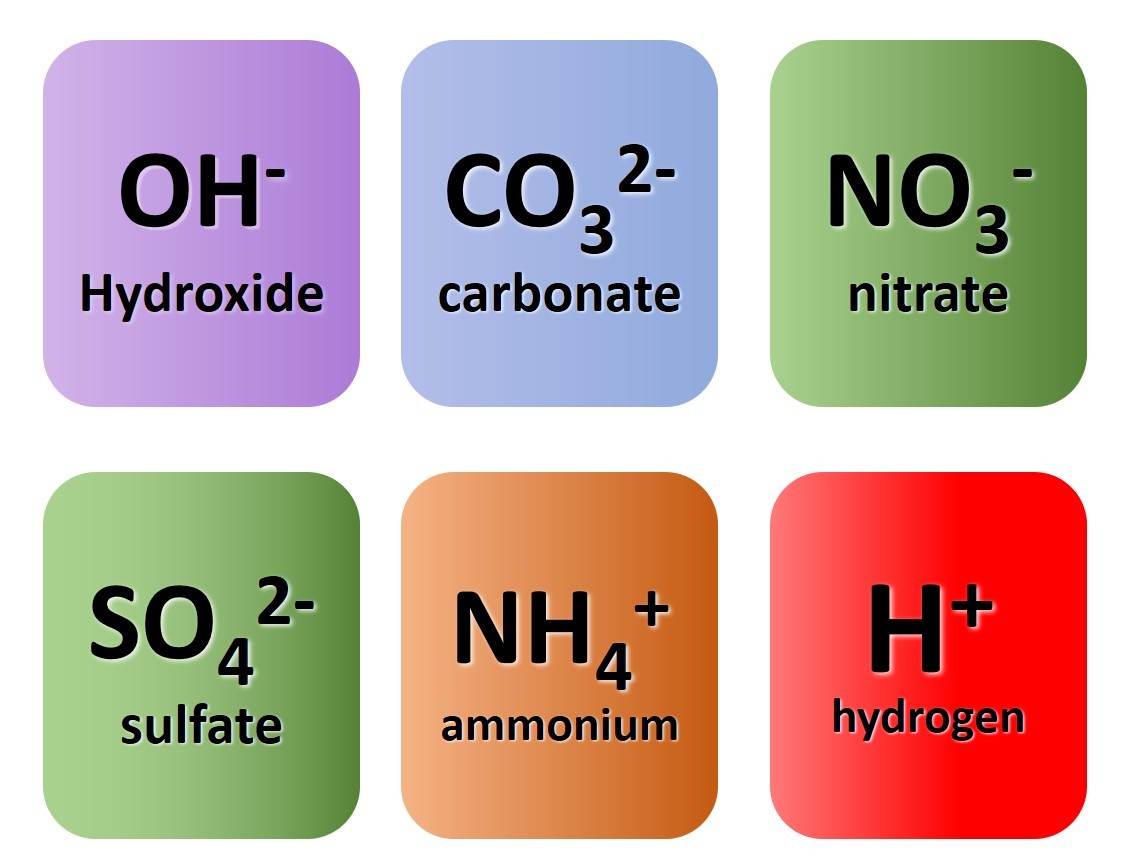
1.39 Activity. Finding formulae Students should: 1.39 write formulae for compounds formed between the ions listed Watch the video closely pausing where instructed. Use the information given to work out the correct formulae for the following compounds: Name of compoundFormula lithium nitridepotassium oxidezinc oxidesilver chlorideLi3N&nb...
Ionic compounds form when the atoms of a metal combine with the atoms of a non - metal