Assumed prior knowledge The Reactivity Series Rust forms on iron and steel as a result of a redox reaction between iron and oxygen. 2.15 - 2.20 Loss and gain Oxidation and redu... https://www.mychem.co.uk/index.php/igcse-chemistry/inorganic-chemistry/reactivity-series OCR Enter your text here ... EDEXCEL AQA Enter your text here ... Oxi...
OCREdexcelAQA Transition elements : properties Ligands and complex ions Ligand substitution and precipitation Redox reactions Electron configurations The video here uses the relevant section of the Royal Society of Chemistry's periodic table website to show the electron configurations of the first row of the transition ...
The trends in first ionisation energies give some important clues about the electron configuration of the elements. What's the trend? First ionisation energies for the first 18 elementsInfogram Periodic patterns General trends: Across a period As the nuclear charge increases so the attraction between the outermost electrons and th...
Before tackling this topic make sure you are able to: explain the difference between intermolecular and intramolecular forces. use electronegativity values to work out dipoles within moleculesuse ideas about molecular shapes and dipoles to predict whether or not a molecule is polar. Hydrogen bonds in DNA&nbs...
Chromatography is a method used for separating ( and identifying) components of a mixture based on how they interact with two phases : 1. Two Phases Stationary phase: stays fixed (solid or a liquid coated on a solid).Mobile phase: moves through the stationary phase (liquid or gas). Separation happens because different substances int...
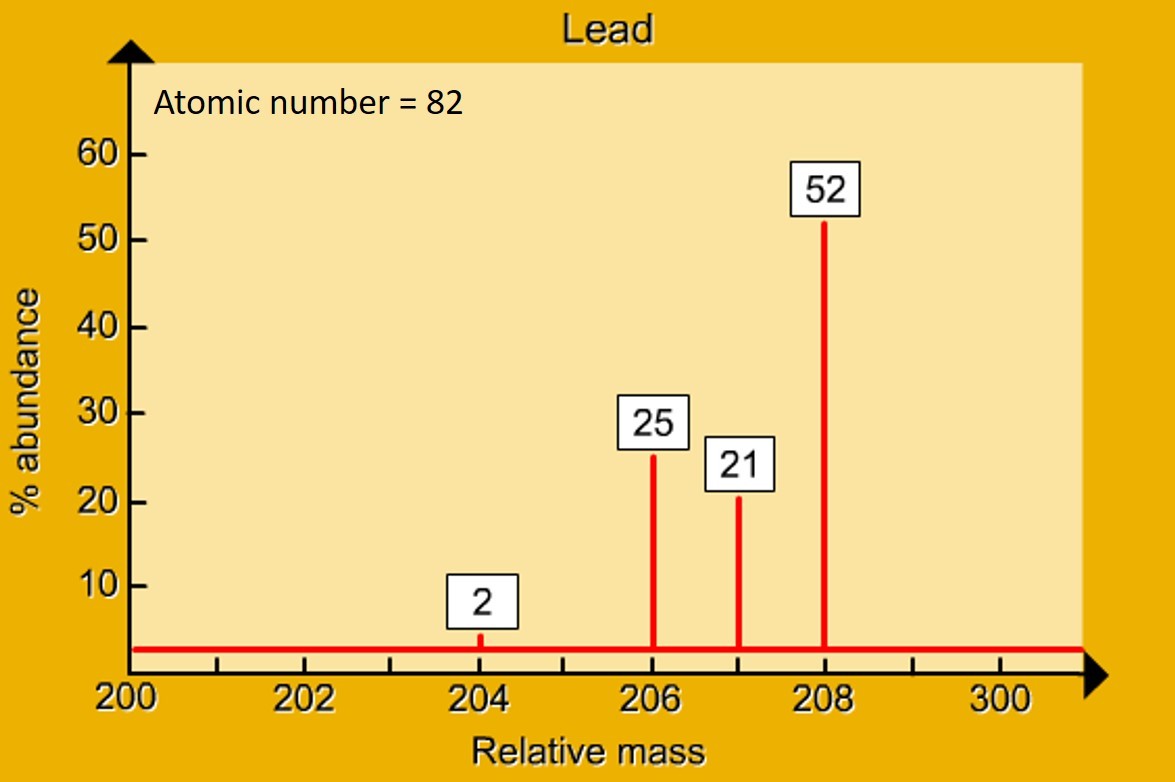
Background 1.14 - 1.17 Atomic structure If a nucleus was the size of a raisin, the rest of the atom would be the size of a sports stadium https://www.mychem.co.uk/index.php/igcse-chemistry/principles-of-chemistry/atomic-structure how it works > A mass spectrometer "sorts" particles according to their mass. A mass spectrum gives the r...
3.17 - 3.22 Reversible reactions and equilibria A question of balance Many chemical reactions can be reversed.&nbs... https://www.mychem.co.uk/index.php/igcse-chemistry/physical-chemistry/equilibria EdexcelOCR Questions The video describes a number of equilibria. Watch the video, pausing as required and answer the questions below: E...
EdexcelOCRAQA Enter your text here ... Enter your text here ... Amino acid Molecule A. This is glycine - the simplest of the amino acids. The systematic name is 2- aminoethanoic acid Amine Molecule B. This is a simple aliphatic amine with two carbon atoms called ethylamine ( or ethanamine) . Ethylamine can be formed...
Proton NMR produces a spectrum with a number of peaks. The number of peaks corresponds to the number of different chemical environments in which protons exist. OCREdexcelAQA Introducing NMR Proton NMR Carbon 13 NMR TMS Looking at the protons High resolution NMR - reveals a splitting pattern. This is produ...
Lithium-ion cells : the Tesla solution This video explains the operation of lithium ion cells as used in Tesla cars. The video highlights the many advantages of the use of lithium in the battery pack. The many advantages of using electric motors to power cars are outlined here. Are there any disadvantages of using elect...