Chemical tests
IntroductionPearson Specification Analytical chemistry involves the identification of chemical substances using a variety of methods. Although modern technology has enabled chemists to analyse and identify a huge range of substances very quickly and accurately some simple test tube reactions can still be quicker and cheaper to perform. 2....
2.34 - 2.43 Acids, alkalis and salt preparation
2.34 Defining salts Common "table" salt ( sodium chloride) is found dissolved in large quantities in seawater. Sodium Chloride is just one example of the many compounds which can be called salts. Most salts are crystalline ionic compounds A salt is defined as : A compound resulting from a chemical reaction of an acid...
2.45 - 2.50 Chemical tests (for ions)
2.45 - 2.46 Up in flames Fireworks produce a whole range of different colours. All fireworks release energy in the form of heat energy and are therefore exothermic. Explosions and rocket power is provided by gunpowder and similar explosive mixtures. Colours are achieved by mixing compounds which contain various metal ions into the explo...
salt preparation
Defining salts Common "table" salt ( sodium chloride) is found dissolved in large quantities in seawater. Sodium Chloride is just one example of the many compounds which can be called salts. Most salts are crystalline ionic compounds A salt is defined as : A compound resulting from a chemical reaction of an acid, in ...
2.28 Acids, alkalis and titrations
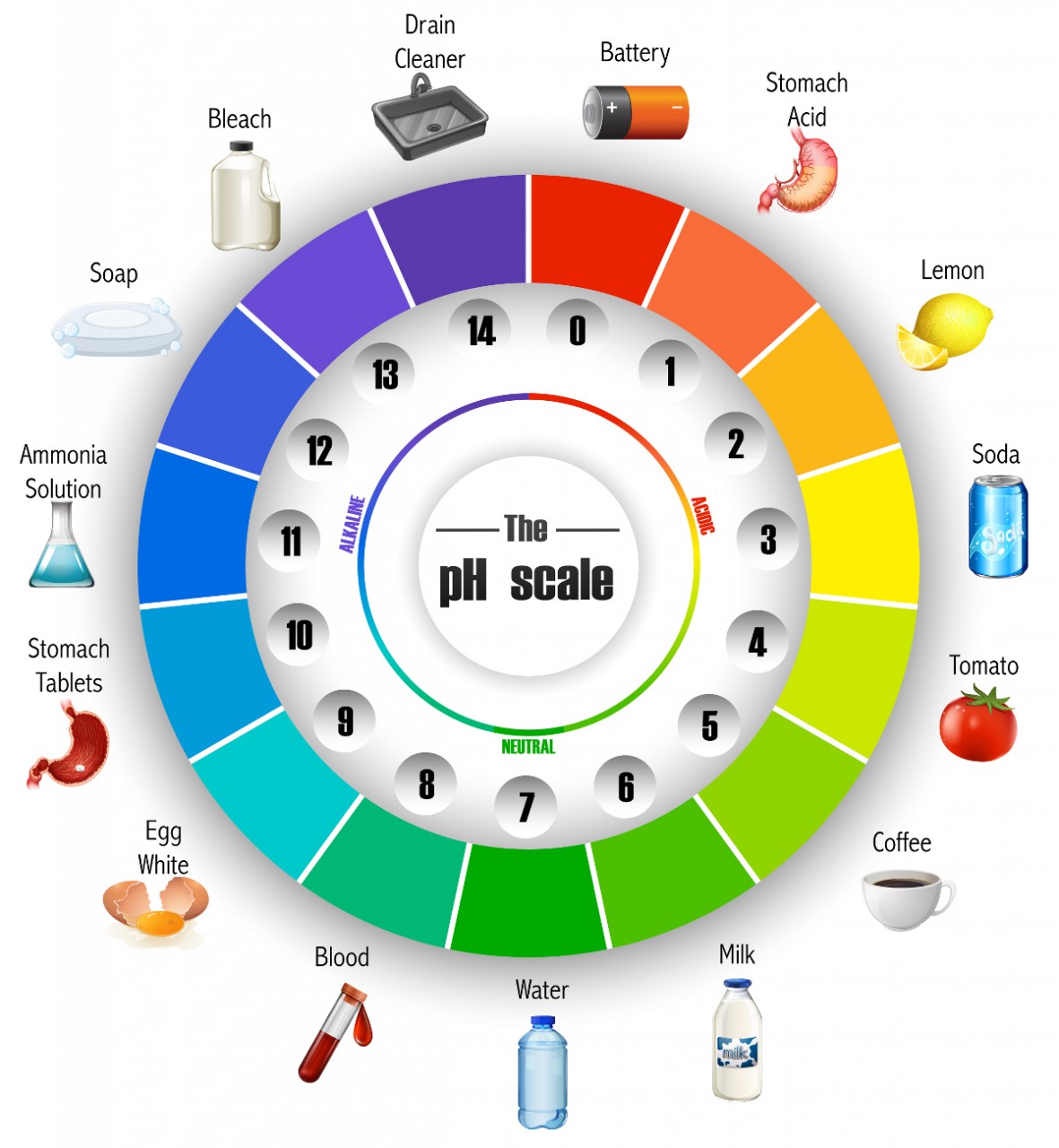
Acids and alkalis are all around us; many everyday foods, drinks, cleaning products are acidic or alkaline. Study the image of the pH scale "wheel". This scale gives a measure of the acidity or alkalinity of a substance. A value of pH 7 is regarded as neutral . Substances with a pH value greater than 7 are regarded as alkaline ...