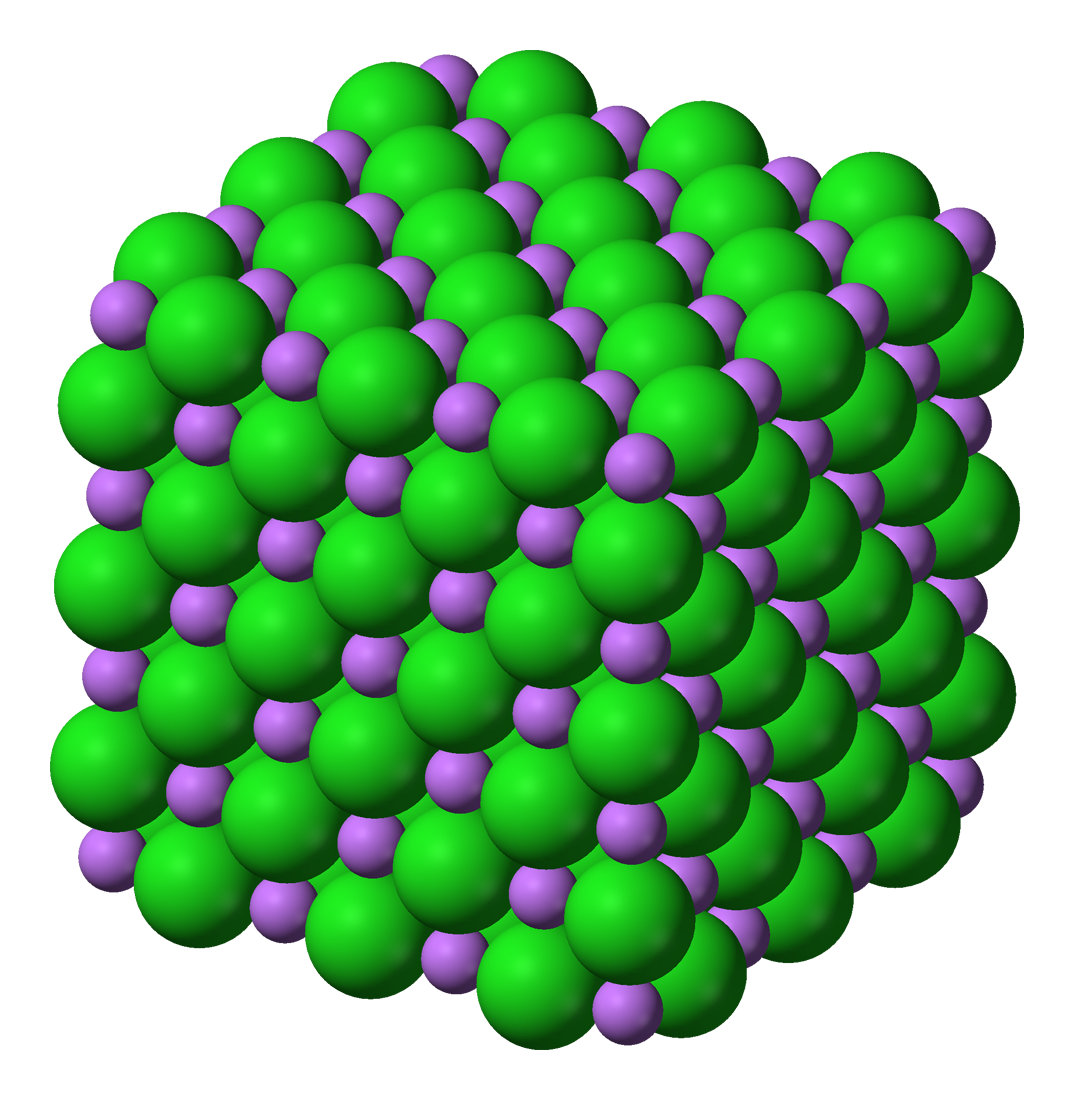
Lattice (Formation) Enthalpy is defined as: The enthalpy change when one mole of an ionic lattice is formed from its constituent ions in a gaseous state. atomisation Enthalpy change when one mole of atoms is formed first ionisation removal of one electron from each atom in one mole of gaseous atoms to form one mole of gaseous ions To answer ...
4.23 - 4.38 The Alkenes Limonene is an alkene which can be found in the skin of citrus fruits 4.23 Meet the alkenes The Alkenes are another Homologous series of Hydrocarbons. They differ from the Alkanes because they contain in their molecules at least one double bond between two of the carbon atoms. Because of their double bonds, t...
Enter your text here ... Amines Intro image Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... https://mychem.co.uk/index.php/home/biology/amines
4.34 - 4.37 From wine to vinegar Carboxylic acids are a homologous series of compounds with the functional group -CO2H. They are all organic acids and are regarded as weak acids which means they do not ionise completely in solution. Carboxylic acids are formed when alcohols are oxidised by the air or by using an appropriate ox...
Sun and air Study the seaside image for a few seconds . Try to describe it: You might say that the image shows : A calm sea, clear blue sky, bright sunshine and a perfectly smooth beach. You could describe it in terms of the states of matter visible: SOLID Sand LIQUID Sea GASAir GAS + PLASMASun But as a chemist y...
Sun and air? Study the seaside image for a few seconds . Try to describe it: You might say that the image shows : A calm sea, clear blue sky, bright sunshine and a perfectly smooth beach. You could describe it in terms of the states of matter visible: SOLID Sand LIQUID Sea GASAirGAS and PLASMASun As a chemistry student you might be...
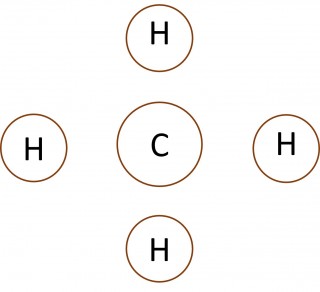
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds. 1.46 Activity 2. Crossing and dotting Students should: 1.46 understand how to use dot-and-cross diagrams to represent covalent bonds in molecules. Dot and cross diagrams are a useful way of representing how atoms share electro...
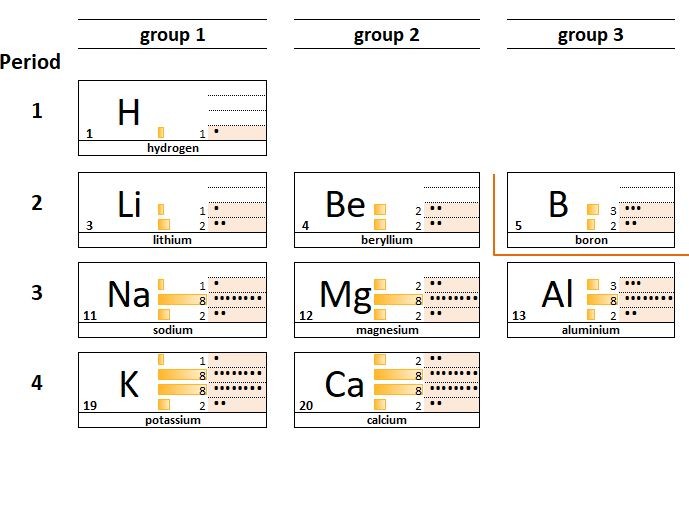
1.23 Family resemblances Hydrogen is often listed above group 1 the Alkali metals. Although like group 1 atoms, hydrogen atoms have a single electron in their outer shell, Hydrogen is not a metal. Students should: 1.23 understand why elements in the same group of the Periodic Table have similarchemical properties 1.24 und...