Physically gases have a number of similarities. For example - all gases will: fill the container in which they are placed and adopt its shapeexpand when heated and contract when cooledbe compressed if subjected to increased pressureHowever despite these physical similarities many gases are chemically very different from one another....
2.45 - 2.46 Up in flames Fireworks produce a whole range of different colours. All fireworks release energy in the form of heat energy and are therefore exothermic. Explosions and rocket power is provided by gunpowder and similar explosive mixtures. Colours are achieved by mixing compounds which contain various metal ions into the explo...
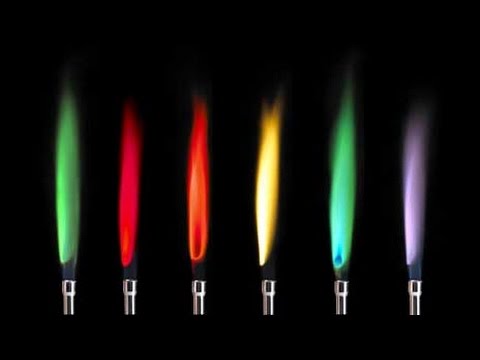
Flame tests can be used to identify some metal ions (cations).Lithium, sodium, potassium, calcium and copper compounds producedistinctive colours in flame tests:• lithium compounds result in a crimson flame• sodium compounds result in a yellow flame• potassium compounds result in a lilac flame• calcium compounds result in an orange-red flame• copper com...
Introduction Water has no obvious smell. Water has no taste and water is colourless. Water has a neutral pH value ( pH 7). Many other chemical substances have similar properties . We therefore need to find a chemiscal test for water. Enter your text here ... When water is added to anhydrous (white) copper sulfate the copper s...
Intro image Enter your text here ... Enter your text here ... To test for carbon dioxide. the test is to force the sample that you believe may be carbon dioxide in to a solution of lime water. if the sample does contain carbon dioxide then the solution will turn cloudy, if it does not then the sample cannot contain carbon dioxide. Enter your text...
Introduction Hydrogen is a flammable gas. It burns in air to produce water. A convenient test for hydrogen is to put a lighted splint in the mouth of a test tube full of the gas . The gas will burn with a characteristic squeaky pop if hydrogen is present. Hydrogen gas can be used as a fuel. When hydrogen burns in air it combines with ox...