1.55 - 1.60 Electrolysis
1.55 Using electrolysis Electrolysis happens when an electric current is passed through an ionic compound which has been melted or dissolved. Electrolysis has a wide range of uses including : electroplating, extraction of aluminium, production of chlorine and the purification of copper . This post helps you to unders...
2.23c - 2.27c Extraction and uses of metals
2.23 Activity 3. Aluminium extraction Electrolysis is used to extract reactive metals from their ores. Aluminium is a good example of this. Work through the animation carefully and then answer the questions below: QuestionsAnswers 1. What ions are present in the compound aluminium oxide? 2. What is the aluminium oxide mixed wi...
1.52 -1.54 Metallic bonding
More than 75% of the elements in the periodic table are metals. Metals are extremely useful materials with a wide variety of uses. This is due to the fact that they have a range of useful physical properties. all metals are : good conductors of heat and electricity most metals are: shiny when freshly cuthigh melting point solidsducti...
1.45 Opposite often attract
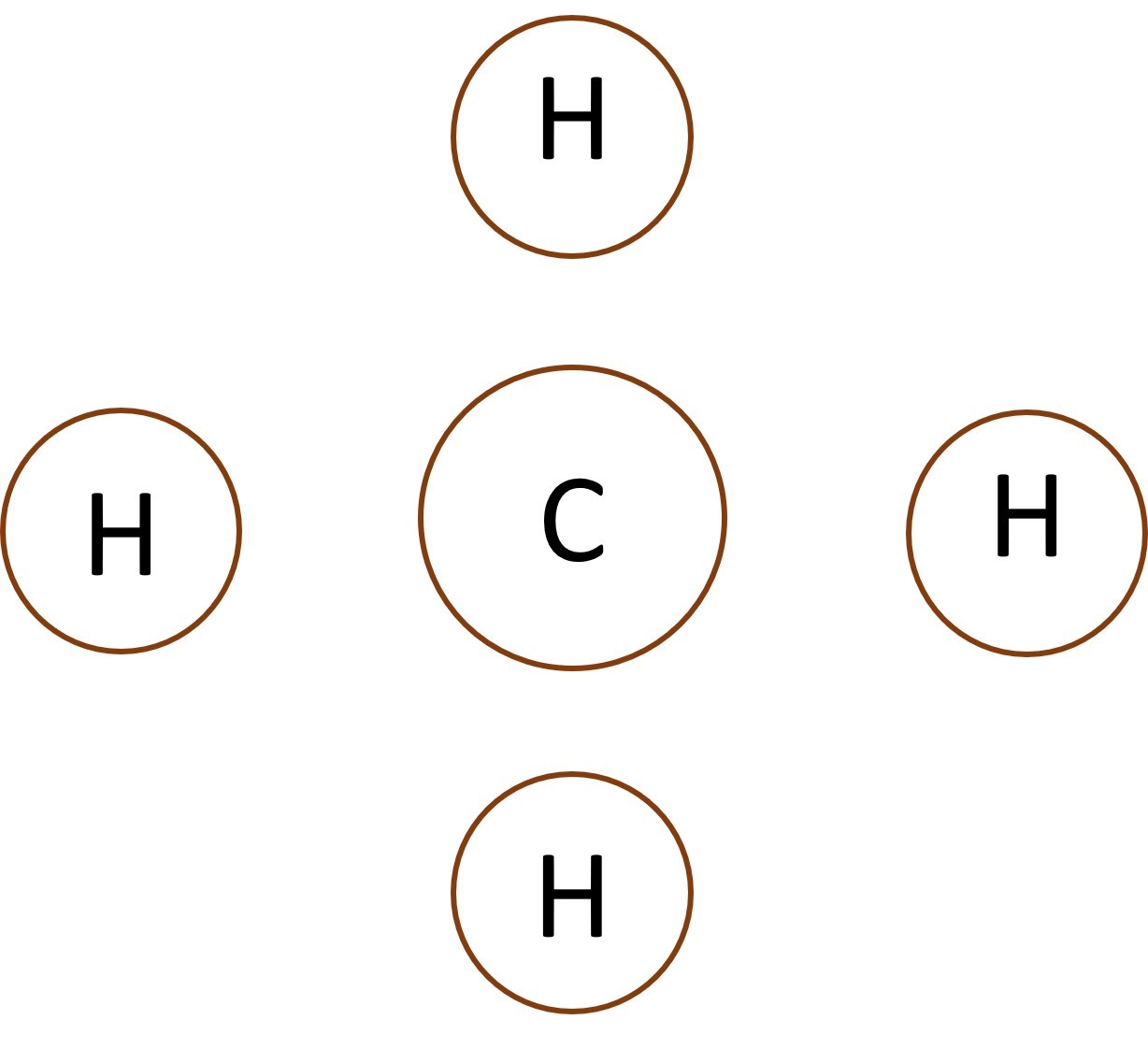
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds. 1.44 - 1.45 Activity. Opposites attract Students should: 1.44 know that a covalent bond is formed between atoms by the sharing of a pair ofelectrons1.45 understand covalent bonds in terms of electrostatic attractions Play the ...
1.44 Covalent bonding - "sharing nicely"-
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds.