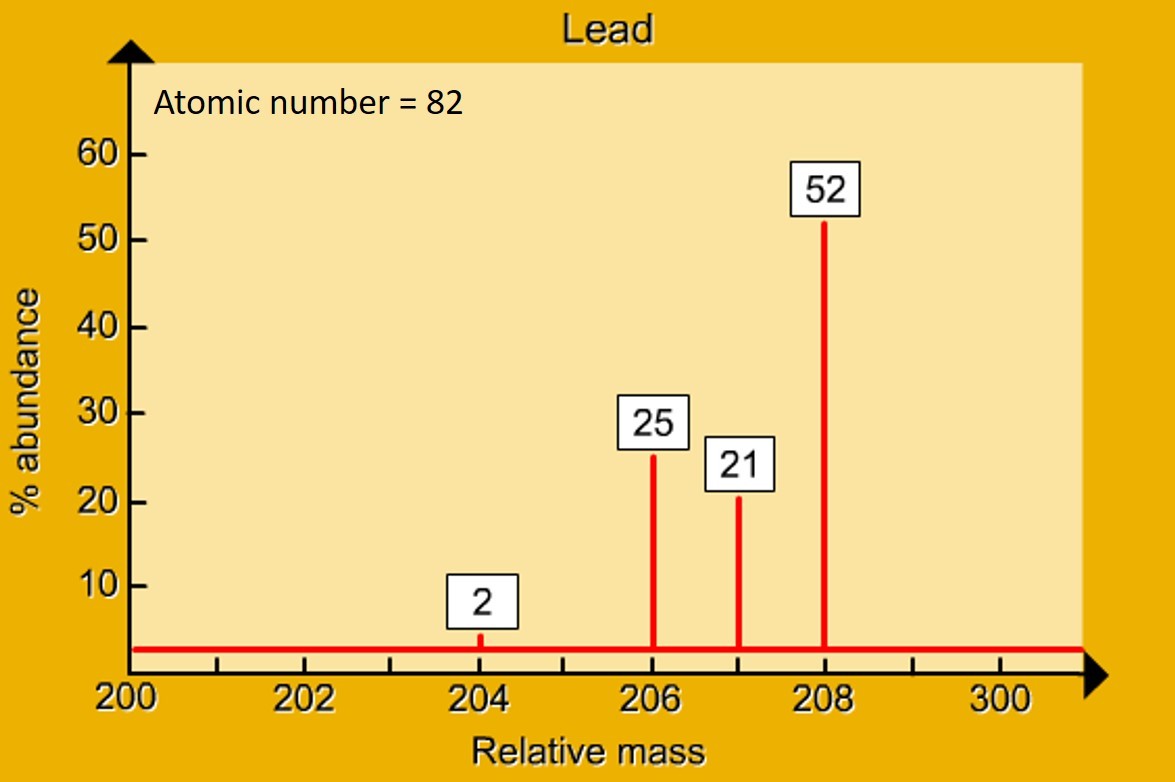
how it works The mass spectrometer "sorts" isotopes according to their mass. It can also be used to analyse organic molecules. Background 1.14 - 1.17 Atomic structure If a nucleus was the size of a raisin, the rest of the atom would be the size of a sports stadium https://www.mychem.co.uk/index.php/igcse-chemistry/principles-of-chemistr...
Physically gases have a number of similarities. For example - all gases will: fill the container in which they are placed and adopt its shapeexpand when heated and contract when cooledbe compressed if subjected to increased pressureHowever despite these physical similarities many gases are chemically very different from one another....
The trends in first ionisation energies give some important clues about the electron configuration of the elements. What's the trend? First ionisation energies for the first 18 elementsInfogram Periodic patterns General trends: Across a period As the nuclear charge increases so the attraction between the outermost electrons and th...
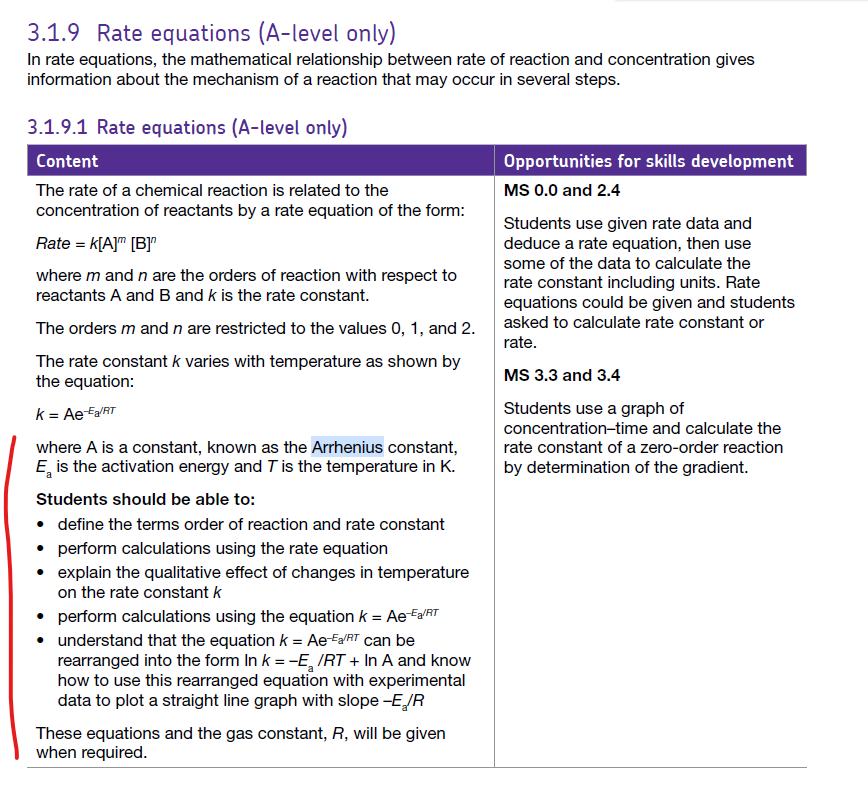
Hot and quick Investigating rates Results The rate of a chemical reaction is significantly affected by its temperature. the effect of temperature on rate can be shown visually using the Maxwell - Boltzmann distribution. OCR Enter your text here ... Edexcel AQA The Maxwell Boltzmann curve is a distribution curve which show...
Look at the images of ice and diamond. In some senses you might say the two substances are similar. Both substances are solid, transparent and crystalline. In terms of bonding, they have similarities too : In ice, atoms of oxygen are held to hydrogen atoms by covalent bonds. In diamond atoms of carbon ar...
Look at the images of ice and diamond. In some senses you might say the two substances are similar. Both substances are solid, transparent and crystalline. In terms of bonding, they have similarities too : In ice, atoms of oxygen are held to hydrogen atoms by covalent bonds. In diamond atoms of carbon ar...
Covalent bonds form when electrons are shared between nuclei Atoms which can form two or more covalent bonds can bond with other similar atoms or themselves and form covalent lattices. Carbon in the form of diamond is a good example, as is silicon dioxide in the form of quartz. A valuable lattice Activity. Diamond - clos...
Intro image Why there might still be hope.. Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ...
Intro image OCROCR 2EdexcelAQA Enter your text here ... Enter your text here ... I had a dream! This presentation outlines the way in which Kekule came to propose his model for the structure of benzene. It also outlines the ways in which the kekule proposal does not adequately explain all the properties of the benzene molecu...
Finding the formula of a compound is important to chemists. We can often predict what the formula might be because we know something about the electron configurations and the way atoms reorganise their electrons to achieve full or empty outer shells. However, here we look at a number of reactions where accurate mass measurement...
In this section we consider how much product is formed by a chemical reaction . Some reaction processes are not very efficient and only a small proportion of the reactants are actually turned into the products. The yield of a chemical reaction is the amount of product obtained. It is usually expressed as a perce...
Intro image Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ...
Students should: 1.10 describe these experimental techniques for the separation of mixtures: simple distillation fractional distillation filtration crystallisation paper chromatography Each technique listed here is a method which can be used for the separation of mixtures of substances. Work through each method in turn . 1.10 Simple distillat...
Relative masses allow chemists to "count out" atoms and molecules so they can ensure that the appropriate amounts of substances are reacted.
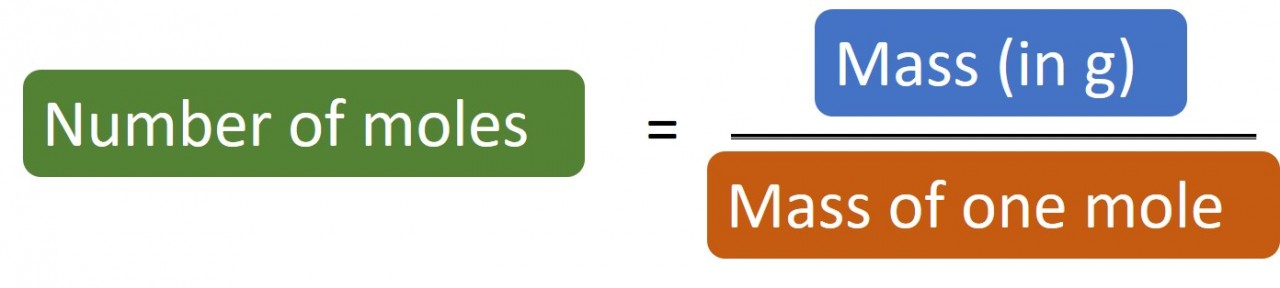
1.28 Calculating moles The following masses of elements all contain one mole of atoms: 12.0 g Carbon, 32.1 g Sulphur, 14 g Nitrogen, 24.3 g Magnesium. So if you have 24 g carbon you have 24/12 = 2 moles carbon atoms. Likewise if you have 1.4 g nitrogen you have 1.4/14 = 0.1 moles nitrogen atoms. Rearrangin...
1.27 What is a mole? Students should: 1.27 know that the mole (mol) is the unit for the amount of a substance 1.28 understand how to carry out calculations involving amount of substance, relativeatomic mass (Ar) and relative formula mass (Mr) A mole (mol) is the quantity of anything that has the same number of particles as there are ato...
1.26 "Counting" by measuring mass Sometimes chemists need to "count" out a specific number of atoms, ions or molecules of a substance. Atoms are very very small - too small to see. It is therefore impossible to measure them out by counting individually. Instead we "count" them out by measuring their mass - much like ...
Relative masses allow chemists to "count out" atoms and molecules so they can ensure that the appropriate amounts of substances are reacted.
Intro image Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ...
To test the effect of particle size on the rate of oxidation we prepared three samples of steel wool each with different levels of coarseness (shown left). The test tube on the left contains the fine steel wool while the one in the middle contains the medium conciseness steel wool and the test tube on the right contains the coarsest stee...