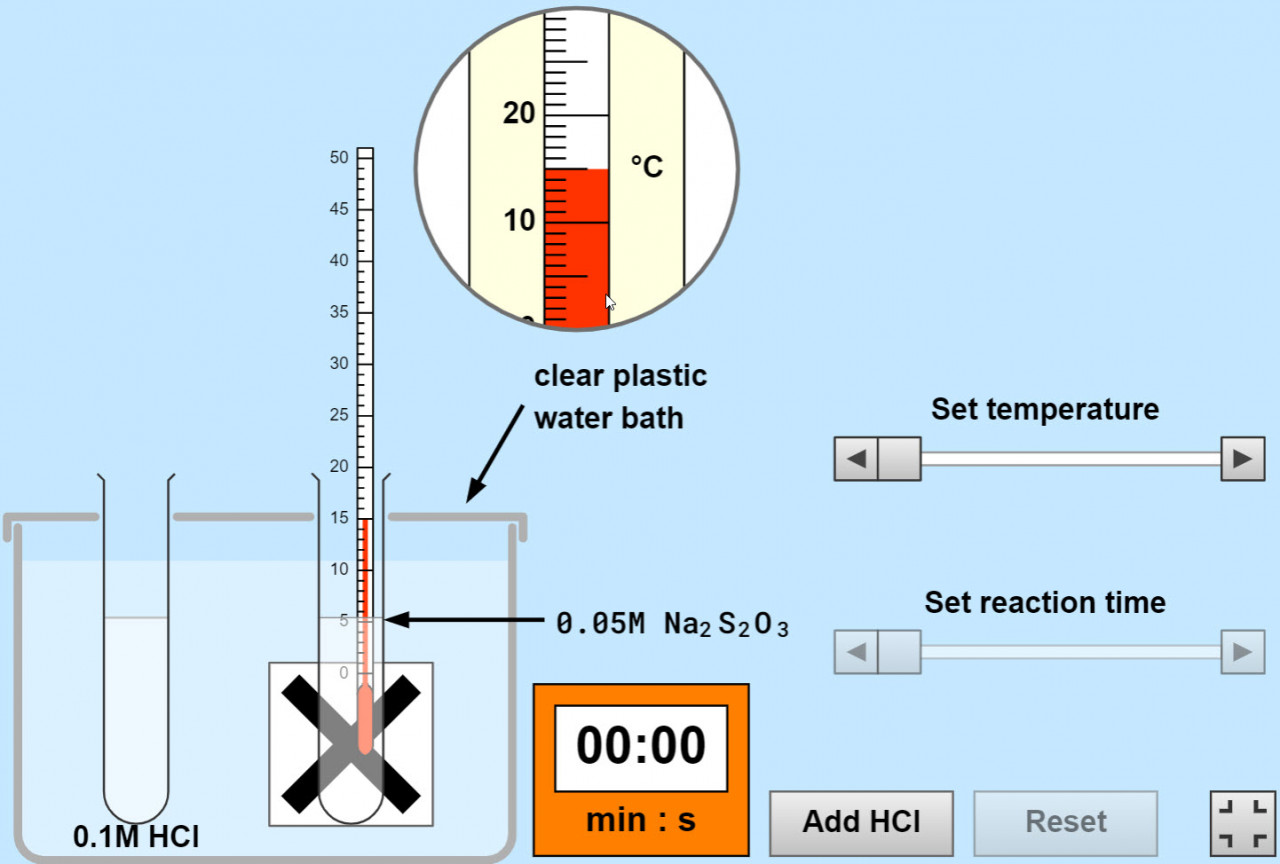
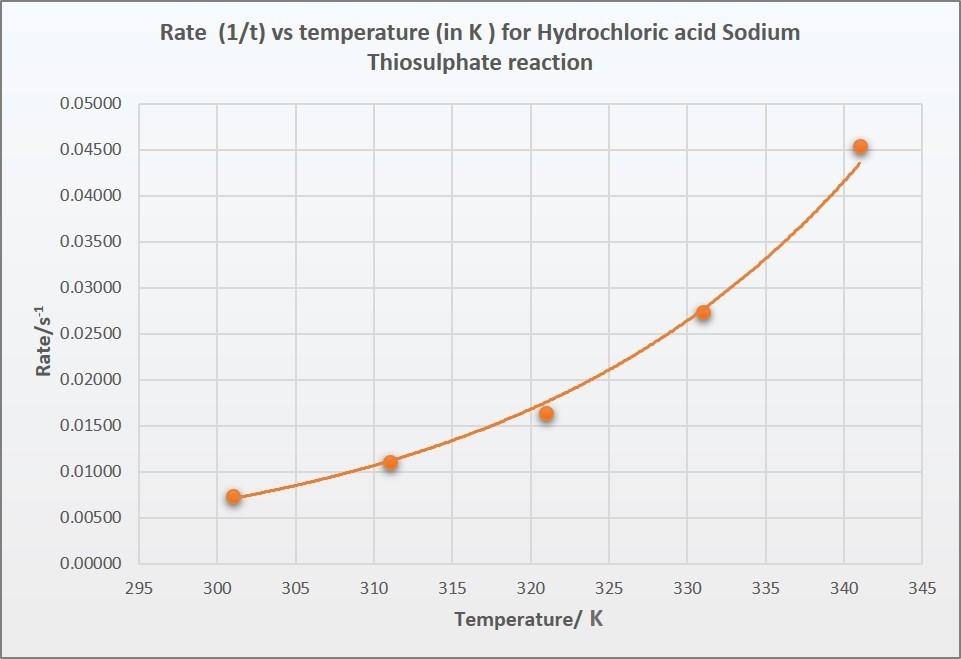
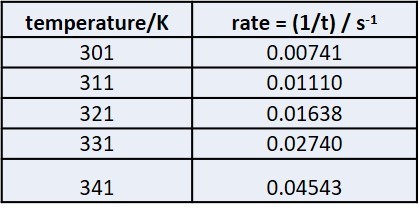
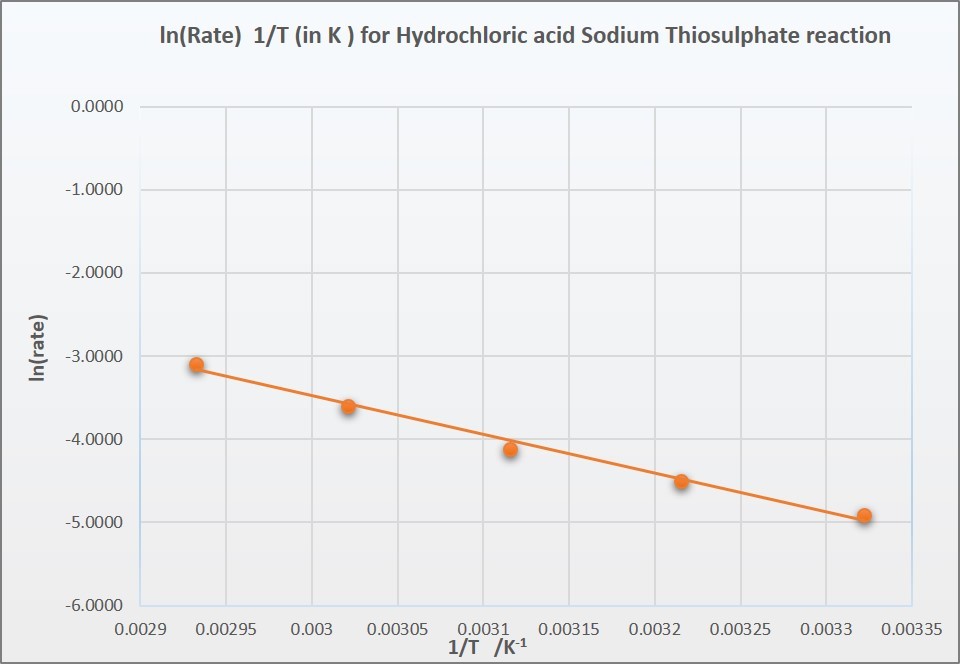
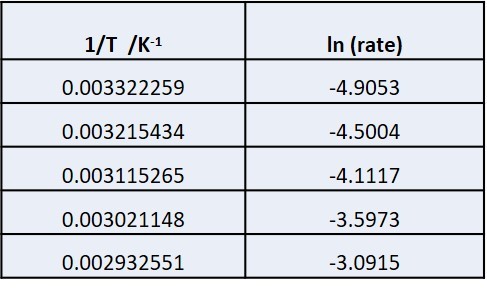
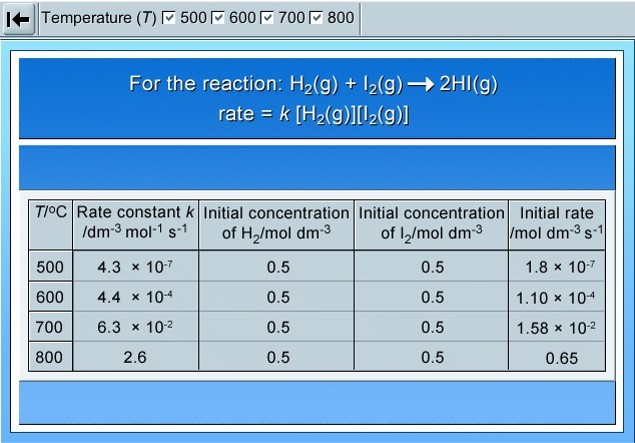
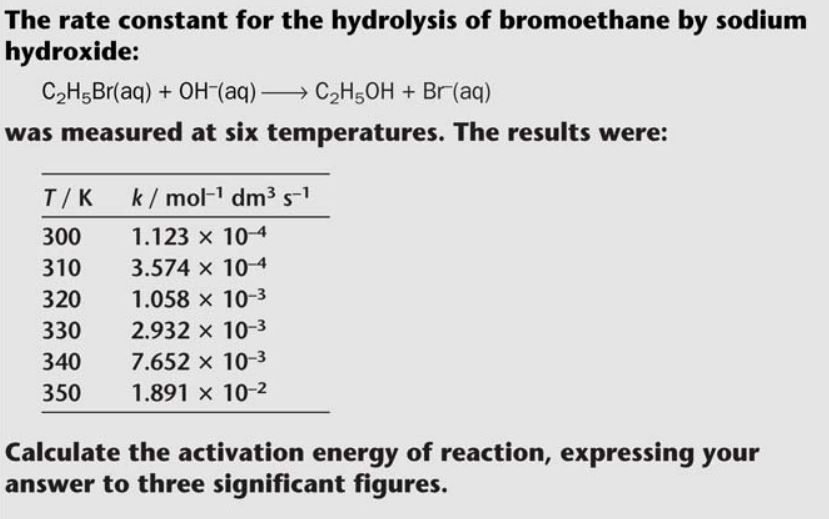
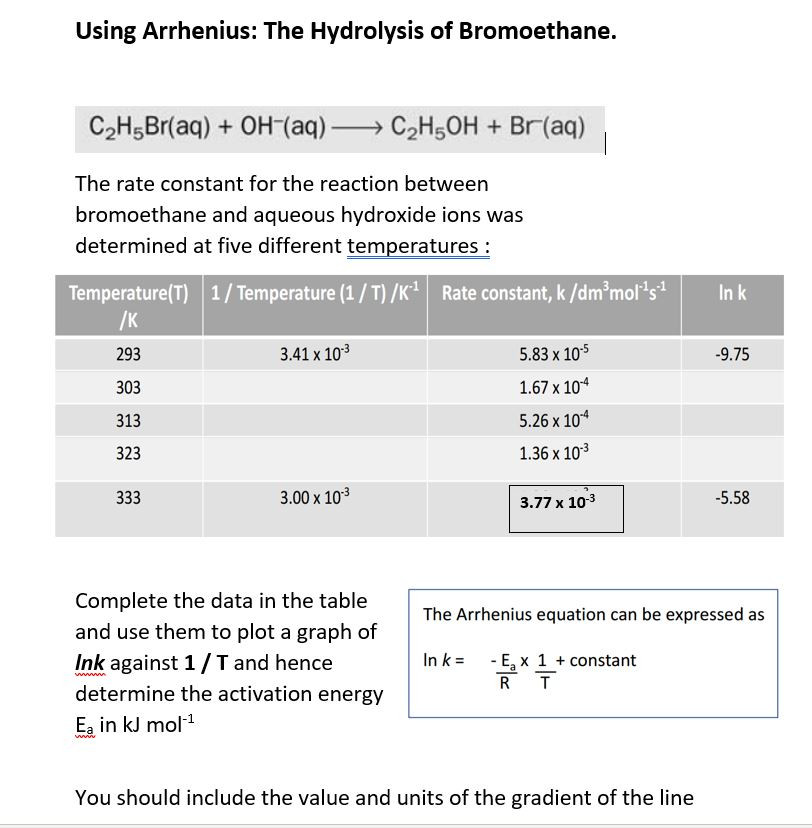
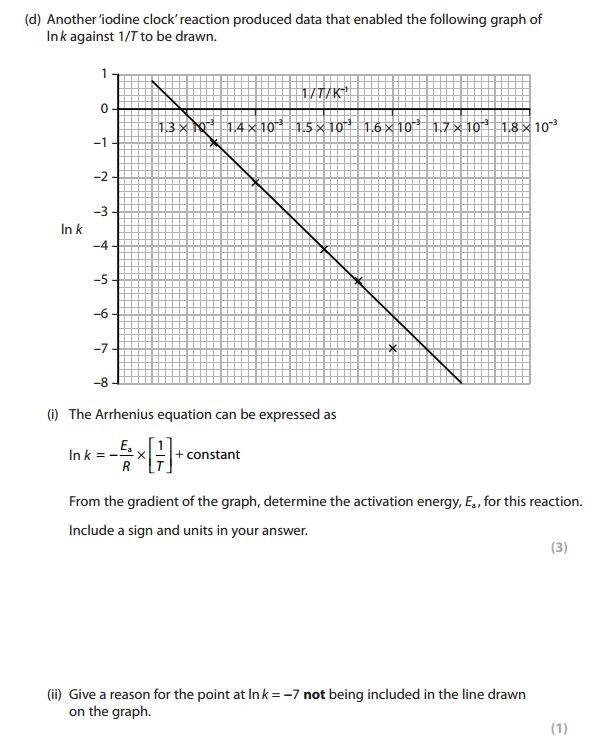
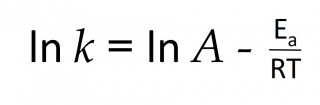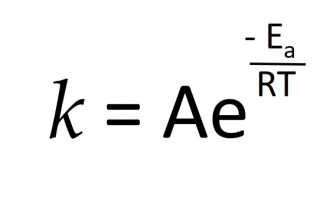Rates and temperature
Hot and quick
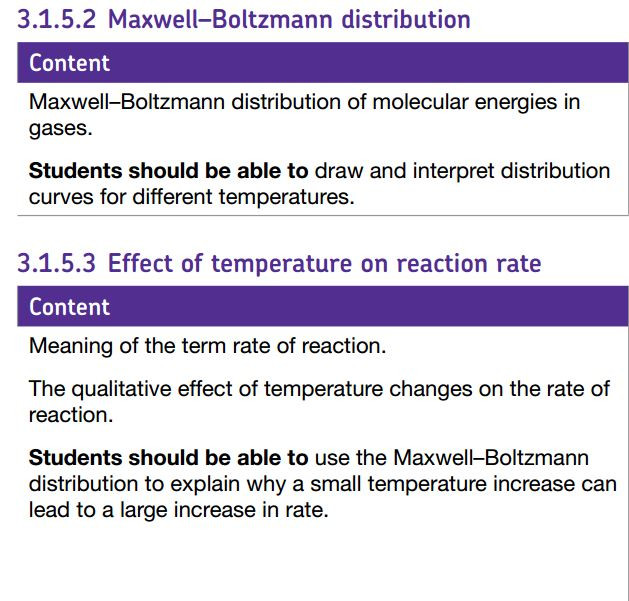
The Maxwell Boltzmann curve is a distribution curve which shows the way in which the speeds of particles in a sample of gas molecules vary.
This video shows how a computer simulation with a limited number of particles produces a distribution curve very similar in shape to the predicted Maxwell- Boltzmann curve.