1. Why is water a liquid and not a gas ( between 0 and 100oC) ? Water has a Relative Molecular mass ( Mr) of 18 ... Oxygen's Mr is 32. If Mr was the only factor that affects the state of a substance then wouldn't you expect water to be a gas ? 2. Why does Ice float on water? Most liquids become more dense as they cool. This caus...
Enter your text here ... Introduction Every chemical reaction has an accompanying change in energy. Some reactions release energy to the surroundings and are known as Exothermic. Reactions which take in energy from their surroundings are known as Endothermic Enter your text here ... This shows a range of chemical changes - all of which rele...
If a nucleus was the size of a raisin, the rest of the atom would be the size of a sports stadium
Atomic models Atoms are the building blocks of matter. Atoms are the smallest possible particles into which an element can be subdivided, without changing its properties. As building blocks, they can be thought of spheres. However, to understand how and why atoms join up with one another, a more sophisticated model is needed....
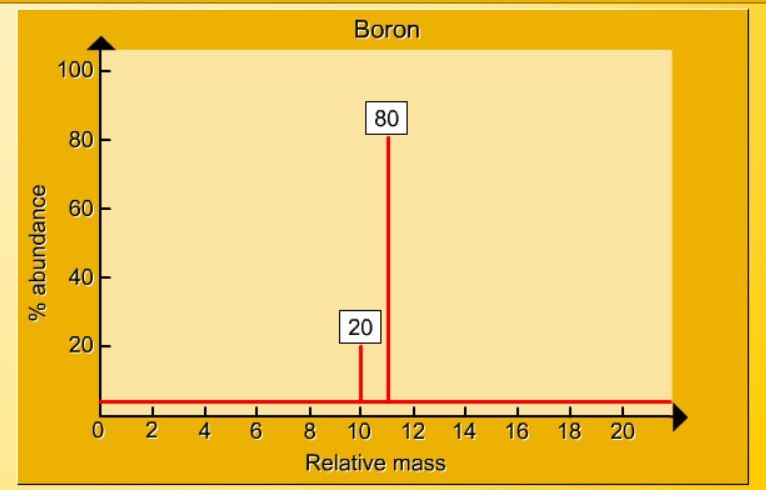
1.17 Activity 2. Calculating relative atomic mass. Students should: 1.17 be able to calculate the relative atomic mass of an element (Ar) from isotopicabundances. Sorting by mass A mass spectrometer is an instrument which can separate and sort the particles in a sample of an element according to their mass. The output from a ...
1.16 Activity 1. Three types of carbon atom Students should: 1.16 know what is meant by the terms atomic number, mass number, isotopes and relative atomic mass (Ar). Use this animation to explore the number and type of sub atomic particles which make up the three isotopes of carbon. Use the information to complete a copy of th...
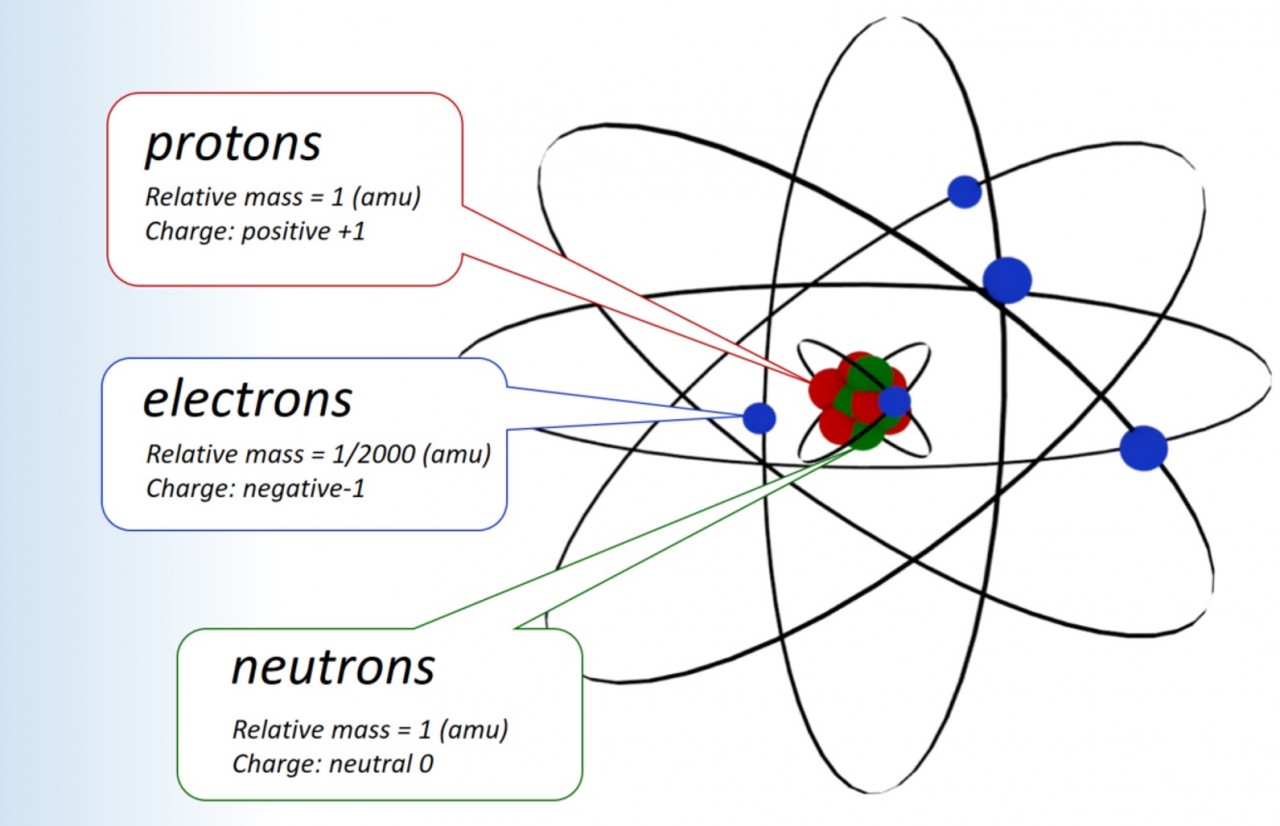
1.15 Sub atomic particles Students should: 1.15 know the structure of an atom in terms of the positions, relative masses and relativecharges of sub-atomic particles. Here we look at the particles inside the atom. Sub atomic particles are the particles found inside atoms. These include protons, neutrons and electrons: protons a...
If a nucleus was the size of a raisin, the rest of the atom would be the size of a sports stadium
reducing your carbon footprint Although this is aimed at students of the IB diploma - it is relevant to all .... Enter your text here ...
Background This topic is mostly about equilibria so it is important for students to understand that concept first. Before tackling this topic make sure you : understand dynamic equilibriacan write and manipulate equilibrium expressions for a range of reactions.can estimate the pH value of a solution using universal indicator know th...
Background You should already know that: • some substances are acidic • the opposite of acidic is alkaline • acids and alkalis can be harmful • we can measure acidity or alkalinity using the pH scale Strong acids When an acid molecule ionises completely when in solution in water, it is regarded as a strong acid. It is said to...
Carboxylic acids can occur naturally : When alcohols are oxidised they can produce a type of acid known as a carboxylic acid. one example of a carboxylic acid is ethanoic acid . This is also known as Acetic acid and is the acid found in vinegar. Other examples of carboxylic acids are methanoic acid ( also known as formic acid) whi...
Many organic molecules can be made to join up with one another to form very long molecules which can be thousands of atoms long. This makes new materials with water resistant properties. Many of these materials will soften when heated and can therefore be easily moulded into complex shape.These materials are known are...
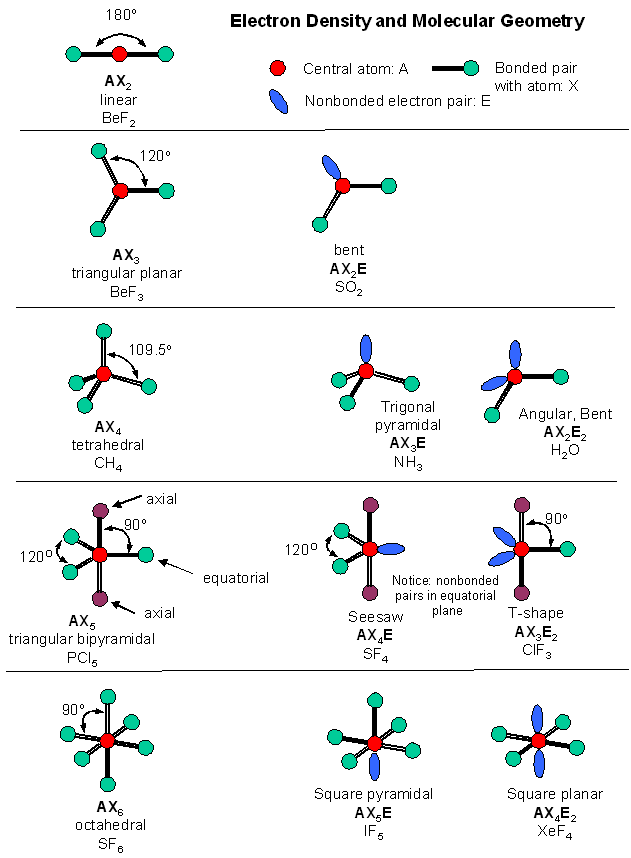
Accounting for electrons. The images in this slideshow here show how we can account for the way in which electrons are shared between atoms. Use the first of the three buttons to the top left of the animation to see the outer electron configuration of each atom. Construct a table to show the name of the molecule, formula, no.of bond...
Modern methods Activity 1 : Phytomining and Bioleaching Watch the video carefully, stopping where necessary to answer the questions below: QuestionsAnswers Explain the main difference between high grade ore and low grade ore.What is happening to the supply of high grade copper ore?Explain what is meant by bioleachin...
1.3 Activity 4. A particular problem Students should: 1.3 understand how the results of experiments involving the dilution of coloured solutions and diffusion of gases can be explained. Particle theory states that all matter consists of many, very small particles which are constantly moving or in a continual state of motion. T...
1.1 Introducing the states Matter can exist in one of three fundamental states: Solid, Liquid or Gas Here we consider the way in which these three states behave We see how matter can be converted from one state into another and we introduce the terms used for these changes. Evaporation, condensation, melting, freezing , sublimation and deposi...
Gases can diffuse into each other . This is because the particles in a gas are in a state of constant random motion. 1.4 Activity 5 : Defining diffusion, diluting and dissolving Gases can diffuse into each other . This is because the particles in a gas are in a state of constant random motion. Liquids can diffuse into other liquids. Whe...