Oxygen is a key component of the atmosphere and is critical for the support of life. Its reactivity makes it essential for respiration and combustion reactions - both of which release energy. Oxides are formed when oxygen combines with other elements. Much of Earth's crust (48.5% by mass) is composed of oxygen in the form of oxides ...
Introduction Group 17 ( used to be group 7) contains a set of non-metals known collectively as The Halogens. Like many families, the members of Halogen family have a number of similarities to each other. However, they also have some very important differences. Activity 1: Ferocious Fluorine This video shows&nbs...
A vertical column the periodic table is known as a group. Group 1 contains six very reactive metals. You might handle lithium and possibly sodium in the laboratory . The other metals in the group are too reactive to handle in a school laboratory. Introduction A vertical column the periodic table is known as a group. Group 1 contains six very ...
Enter your text here ... Enter your text here ... Enter your text here ...
This is a test to see if the glossary works with Easyblog A metal atom . Enter your text here ... Enter your text here ...
1.55 Using electrolysis Electrolysis happens when an electric current is passed through an ionic compound which has been melted or dissolved. Electrolysis has a wide range of uses including : electroplating, extraction of aluminium, production of chlorine and the purification of copper . This post helps you to unders...
2.23 Activity 3. Aluminium extraction Electrolysis is used to extract reactive metals from their ores. Aluminium is a good example of this. Work through the animation carefully and then answer the questions below: QuestionsAnswers 1. What ions are present in the compound aluminium oxide? 2. What is the aluminium oxide mixed wi...
More than 75% of the elements in the periodic table are metals. Metals are extremely useful materials with a wide variety of uses. This is due to the fact that they have a range of useful physical properties. all metals are : good conductors of heat and electricity most metals are: shiny when freshly cuthigh melting point solidsducti...
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds. 1.44 - 1.45 Activity. Opposites attract Students should: 1.44 know that a covalent bond is formed between atoms by the sharing of a pair ofelectrons1.45 understand covalent bonds in terms of electrostatic attractions Play the ...
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds.
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds. 1.45 Activity. Opposites attract Students should: 1.44 know that a covalent bond is formed between atoms by the sharing of a pair ofelectrons1.45 understand covalent bonds in terms of electrostatic attractions Play the video t...
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds.
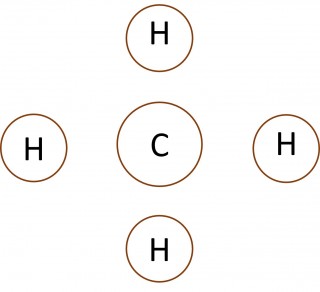
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds. 1.46 Activity 2. Crossing and dotting Students should: 1.46 understand how to use dot-and-cross diagrams to represent covalent bonds in molecules. Dot and cross diagrams are a useful way of representing how atoms share electro...
Magnesium is a highly reactive metal which reacts violently with oxygen to produce a white solid called magnesium oxide. This can be shown as follows: Chemical equations are a useful shorthand way of describing the changes that take place when a chemical reaction happens. Word equations can be helpful for example :...
Use the animation to measure the bond angles in each of the three molecules below:
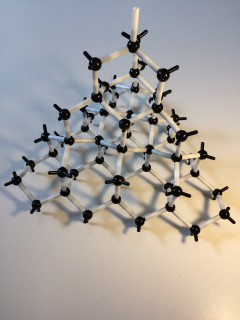
in this topic, we have looked at the way in which substances form different structures. The type of structure formed depends on the type of bonding: Three types of bonding are. metallic, covalent and ionic. metallic: formed when metals bond with themselves or other metals. covalent: formed when non-metal bonds with itself or another...