This problem looks at Kp and the values of partial pressures for Sulphur dioxide and oxygen Enter your text here ...
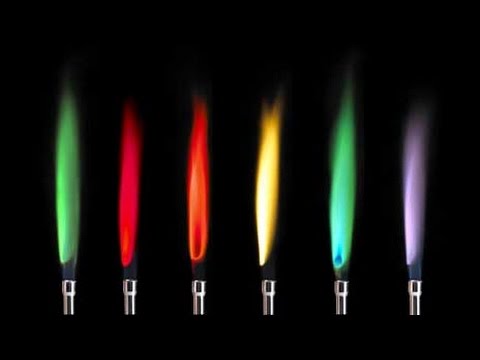
Flame tests can be used to identify some metal ions (cations).Lithium, sodium, potassium, calcium and copper compounds producedistinctive colours in flame tests:• lithium compounds result in a crimson flame• sodium compounds result in a yellow flame• potassium compounds result in a lilac flame• calcium compounds result in an orange-red flame• copper com...
Lithium-ion cells : the Tesla solution This video explains the operation of lithium ion cells as used in Tesla cars. The video highlights the many advantages of the use of lithium in the battery pack. The many advantages of using electric motors to power cars are outlined here. Are there any disadvantages of using elect...
Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ...
Enter your text here ... Enter your text here ... Enter your text here ...
Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Sometimes in organic synthesis there is a need to extend the length of a carbon chain . This can be achieved in a few different ways:
Enter your text here ... The halogens are a family of elements in group 7 (aka 17) of the periodic table. in this video, you can see how reactive the halogens can be. you can also see the characteristic colours of: chlorine; a pale green gasbromine; a volatile orange-brown liquid.iodine; a shiny grey metallic-looking solid with a ...
Proton NMR produces a spectrum with a number of peaks. The number of peaks corresponds to the number of different chemical environments in which protons exist. OCREdexcelAQA Introducing NMR Proton NMR Carbon 13 NMR TMS Looking at the protons High resolution NMR - reveals a splitting pattern. This is produ...
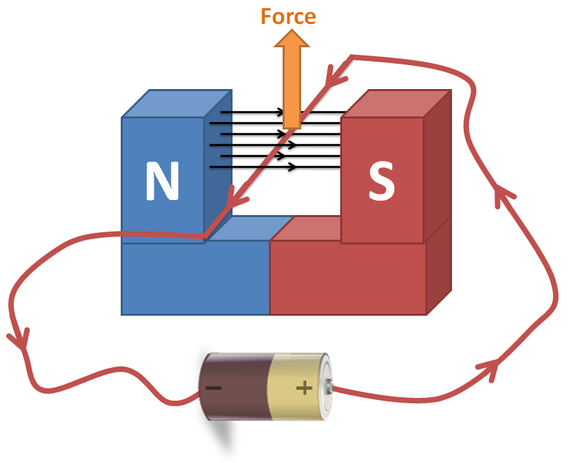
The motor effect When a current passes through a conductor in a magnetic field, the conductor experiences a force. This force is used to provide the motion which drives electric motors. It is therefore known as the Motor Effect Enter your text here ... Enter your text here ...
the alkanes are a family of hydrocarbons. hydrocarbons are compounds containing hydrogen and carbon only. methane is the simplest hydrocarbon with the formula CH4. the rest of the "family" (aka: homologous series) is below: Methane CH4Ethane C2H6Propane C3H8Butane C4H10
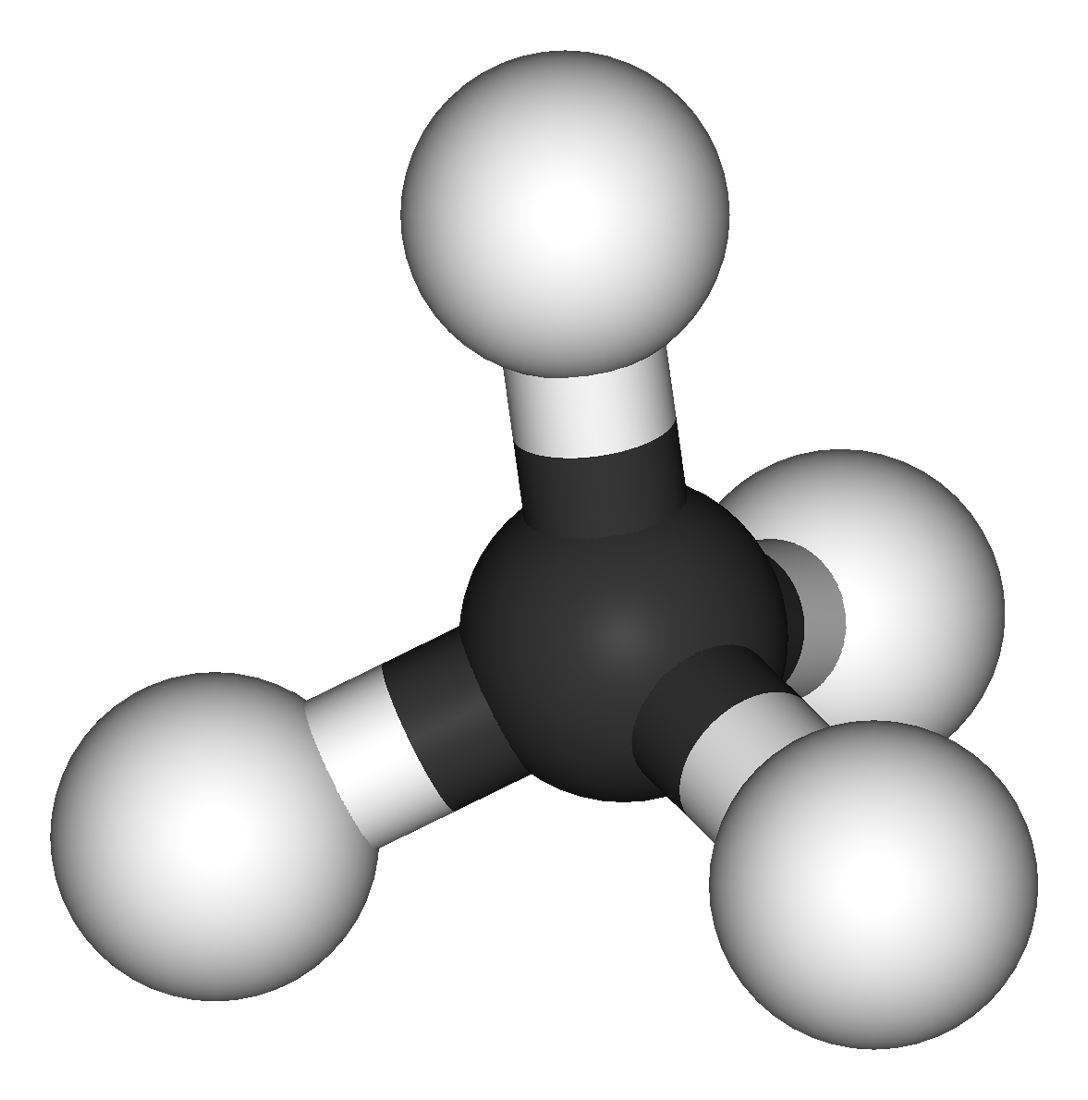
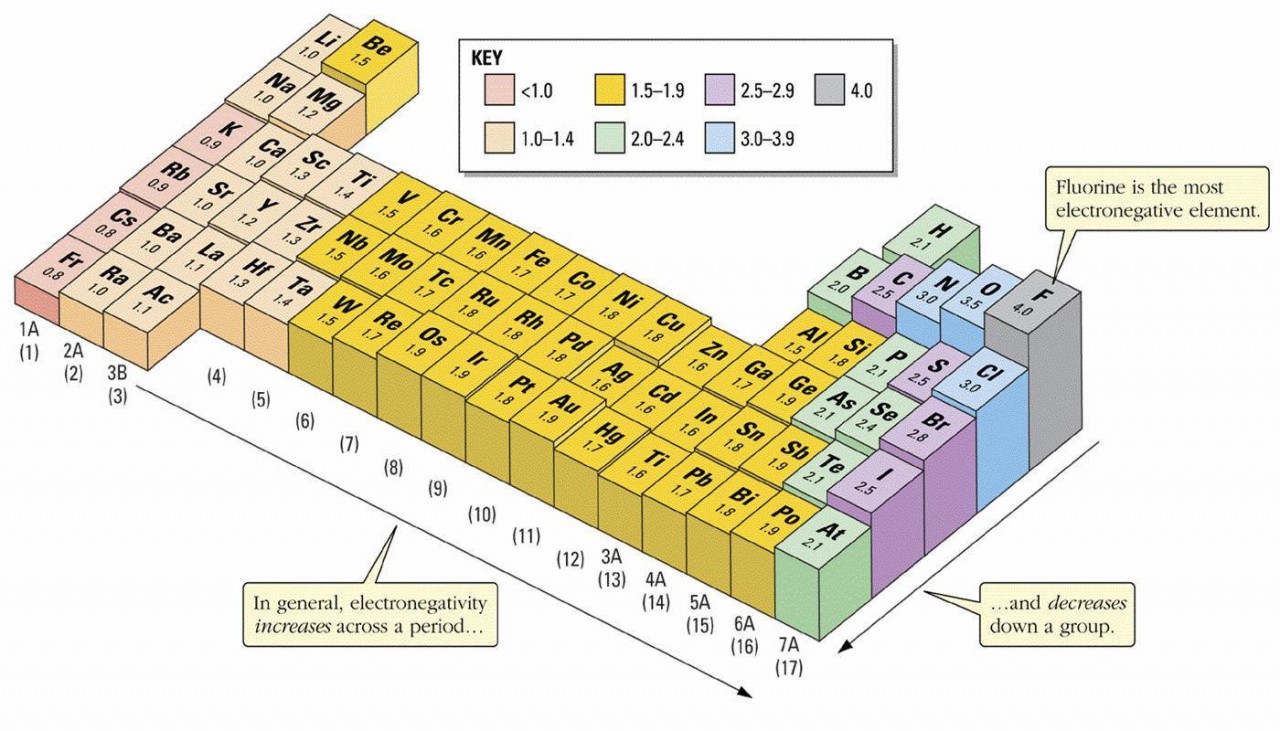
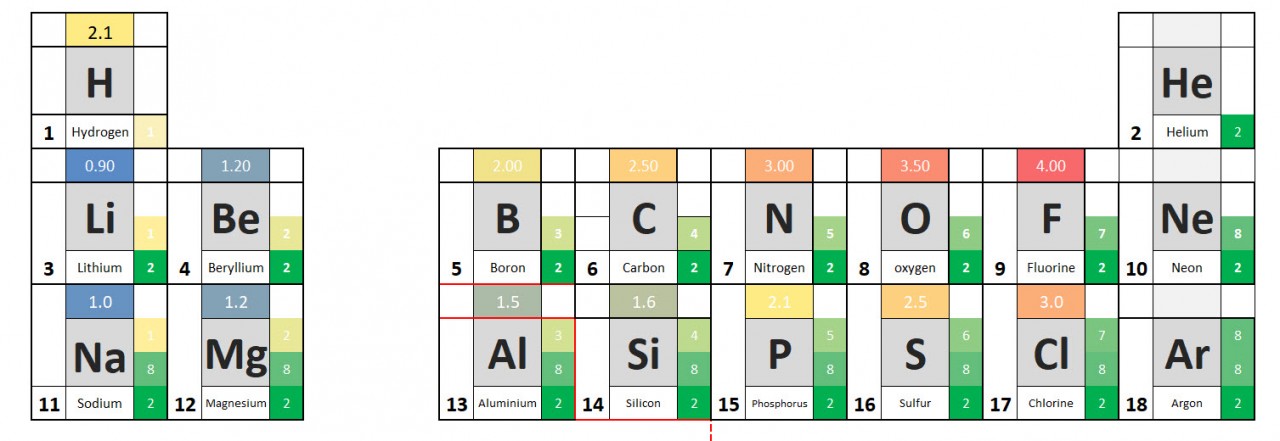
Electronegativity values allow us to predict the direction and strength of dipoles which might exist in molecules
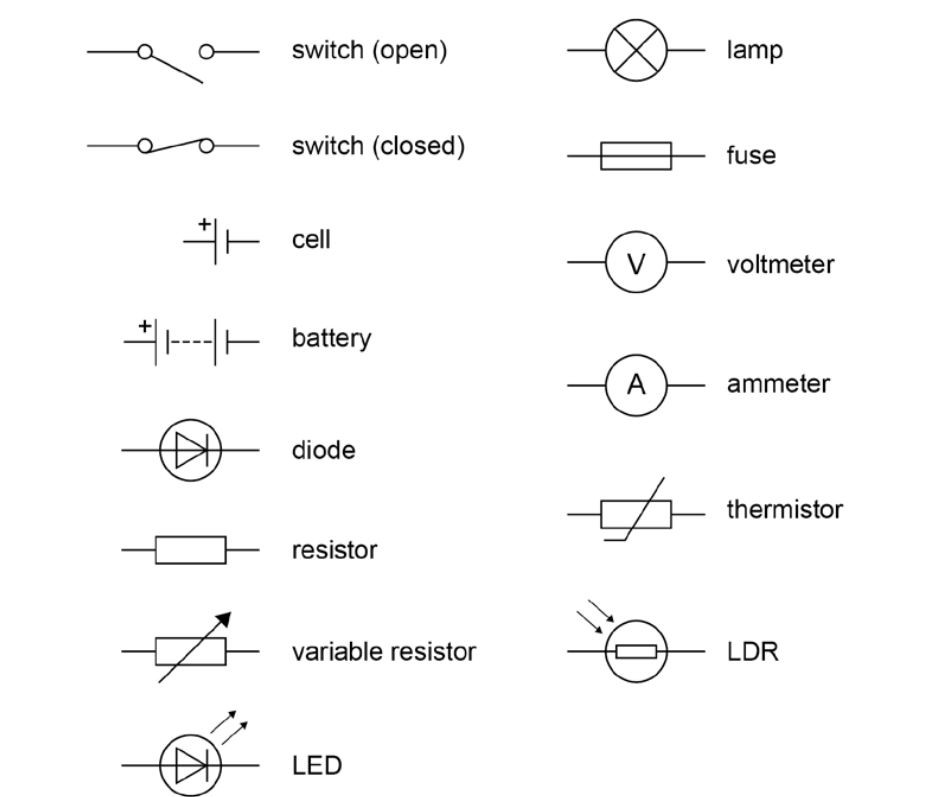
Symbols used in electrical circuit diagrams: Enter your text here ... Enter your text here ...
1.34 understand that ionic compounds have high melting and boiling points because of strong electrostatic forces between oppositely charged ions1.35 understand the relationship between ionic charge and the melting point and boiling point of an ionic compound1.36 describe an ionic crystal as a giant three-dimensional lattice structure held together...
Ionic compounds form when the atoms of a metal combine with the atoms of a non - metal
Ionic compounds form when the atoms of a metal combine with the atoms of a non - metal
1.38 Activity. Know your ions. Students should: 1.38 know the charges of common ions listed You will have noted from the video that metal atoms tend to lose the electrons in their outermost electrons and form positive ions. The charge of the ion formed is equal to the number of electrons lost. Non metal atoms gain el...
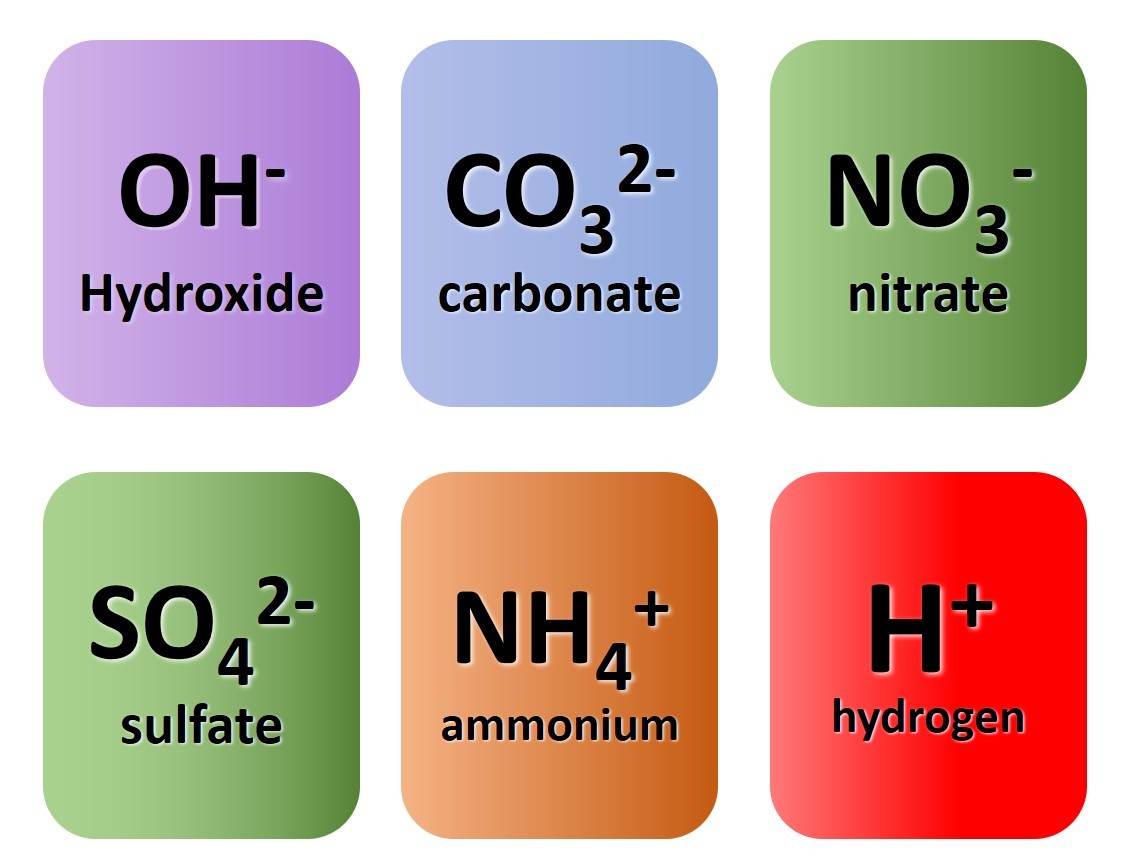
1.39 Activity. Finding formulae Students should: 1.39 write formulae for compounds formed between the ions listed Watch the video closely pausing where instructed. Use the information given to work out the correct formulae for the following compounds: Name of compoundFormula lithium nitridepotassium oxidezinc oxidesilver chlorideLi3N&nb...
2.15 - 2.20 Loss and gain Oxidation and reduction reactions are very commonplace. When a element combines with oxygen that element is said to have been oxidised. When the oxygen is removed from an oxide of an element to leave the element on its own, the oxide is said to have been reduced. Here we will use broader definition of oxidation ...
Before tackling this topic make sure you are able to: explain the difference between intermolecular and intramolecular forces. use electronegativity values to work out dipoles within moleculesuse ideas about molecular shapes and dipoles to predict whether or not a molecule is polar. Hydrogen bonds in DNA&nbs...