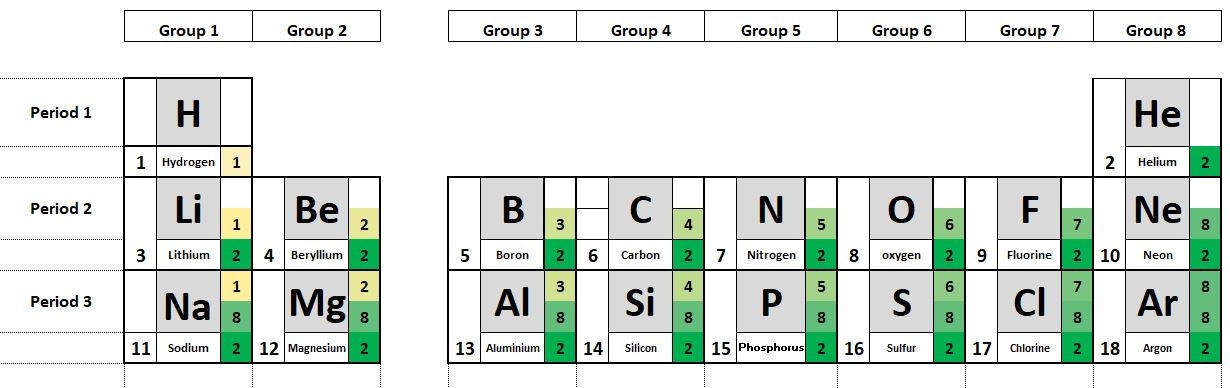
The elements in the periodic table are organised in order of increasing atomic number.
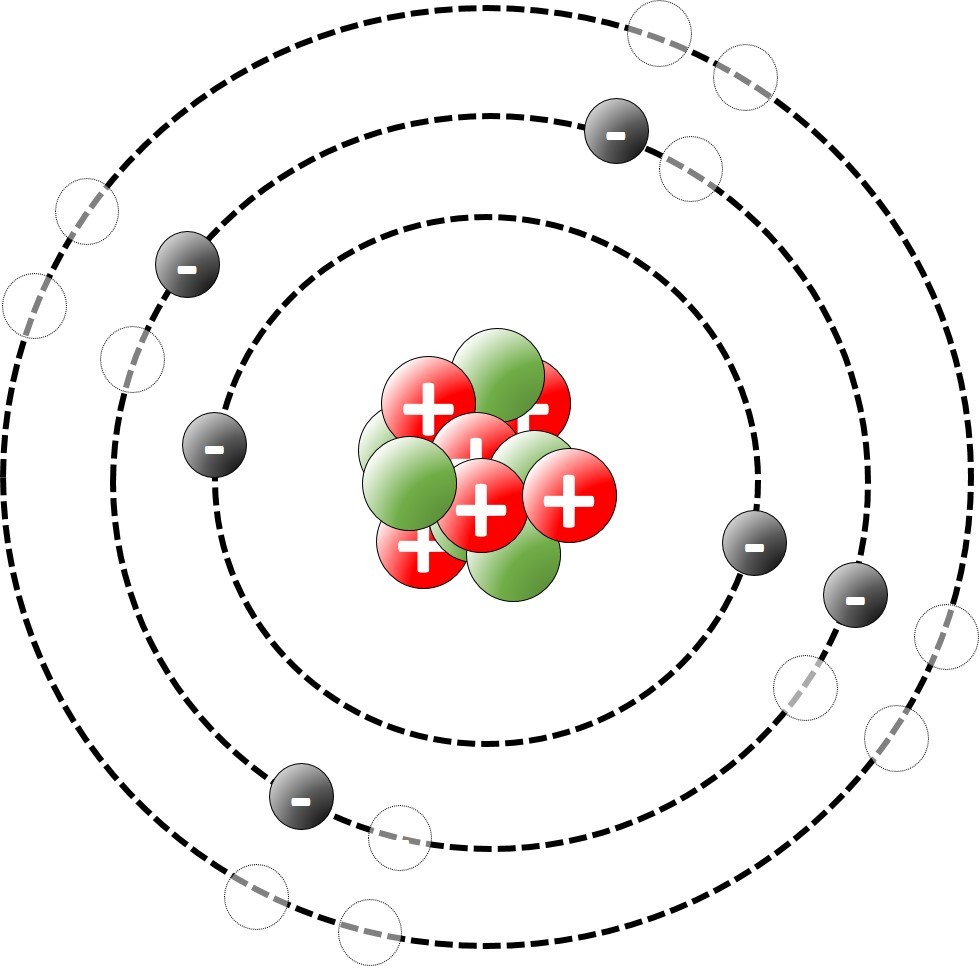
1.19 Deducing electron configurations Students should: 1.19 understand how to deduce the electronic configurations of the first 20 elements fromtheir positions in the Periodic Table A carbon atom has 6 protons and therefore 6 electrons. The electrons are arranged in two shells; 2 electrons in the first shell and 4 electrons in...
1.20 - 1.22 Activity 2. Periodic variations Students should: 1.20 understand how to use electrical conductivity and the acid-base character of oxides to classify elements as metals or non-metals 1.21 identify an element as a metal or a non-metal according to its position in the Periodic Table 1.22 understand how the electronic configura...
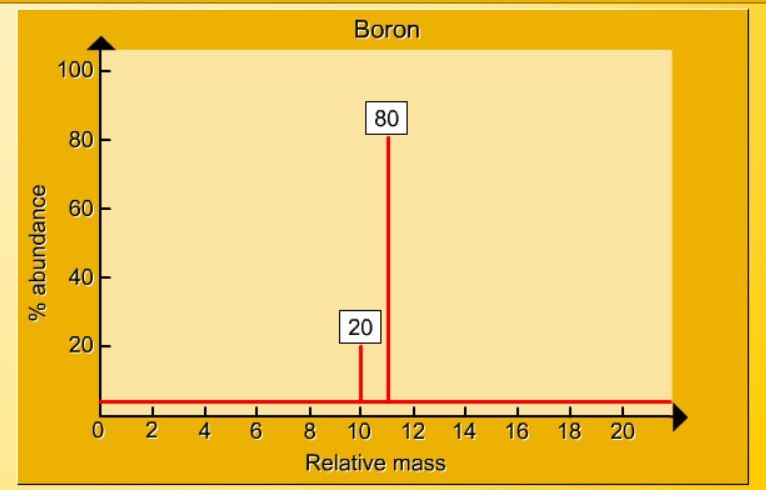
1.17 Activity 2. Calculating relative atomic mass. Students should: 1.17 be able to calculate the relative atomic mass of an element (Ar) from isotopicabundances. Sorting by mass A mass spectrometer is an instrument which can separate and sort the particles in a sample of an element according to their mass. The output from a ...
1.16 Activity 1. Three types of carbon atom Students should: 1.16 know what is meant by the terms atomic number, mass number, isotopes and relative atomic mass (Ar). Use this animation to explore the number and type of sub atomic particles which make up the three isotopes of carbon. Use the information to complete a copy of th...
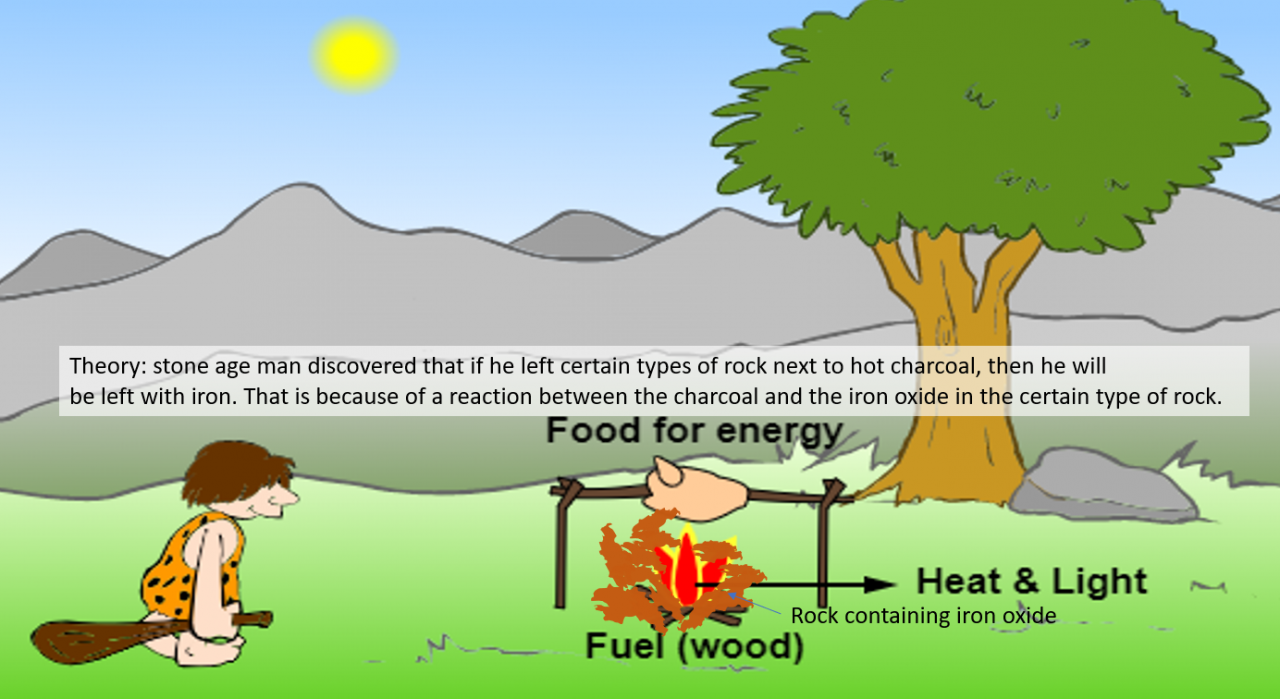
We think that stone age / bronze age man discovered iron by accident . Man must have used fire and probably had a firepit which was used every day. Over time charcoal ( which is mainly carbon) would have built up. Ancient man probably used rocks to contain his firepit. It seems possible that some of these rocks were iron bearing rock...
Colourful compounds Many transition metals have ions which have strong characteristic colours. For example: copper and iron. The metal copper, and iron (rust) have orange like colours. Copper ions are often blue. Blood is red, emeralds are green and the Statue of Liberty is ...? For emeralds, the colour is due to the presence ...
If a nucleus was the size of a raisin, the rest of the atom would be the size of a sports stadium
2.45 - 2.46 Up in flames Fireworks produce a whole range of different colours. All fireworks release energy in the form of heat energy and are therefore exothermic. Explosions and rocket power is provided by gunpowder and similar explosive mixtures. Colours are achieved by mixing compounds which contain various metal ions into the explo...
More than 75% of the elements in the periodic table are metals. Metals are extremely useful materials with a wide variety of uses. This is due to the fact that they have a range of useful physical properties. all metals are : good conductors of heat and electricity most metals are: shiny when freshly cuthigh melting point solidsducti...