4.19 Some saturated hydrocarbons The Alkane series is a family of hydrocarbons. The technical name for this sort of "family" is a "homologous series" The alkane molecules contain atoms of hydrogen and carbon only. The general formula for the alkanes is CnH2n+2 Assumed background knowledge 4.1 - 4.7 O...
Esters are a family of compounds which can be formed as a result of the reaction between a carboxylic acid and an and alcohol. Here you will find out how the reaction is performed and how to draw and name the product.
Sun and air? Study the seaside image for a few seconds . Try to describe it: You might say that the image shows : A calm sea, clear blue sky, bright sunshine and a perfectly smooth beach. You could describe it in terms of the states of matter visible: SOLID Sand LIQUID Sea GASAirGAS and PLASMASun But as a chemist you might be as...
Crude oil is a major source of raw material for the petrochemical industry.
Carboxylic acids can occur naturally : When alcohols are oxidised they can produce a type of acid known as a carboxylic acid. one example of a carboxylic acid is ethanoic acid . This is also known as Acetic acid and is the acid found in vinegar. Other examples of carboxylic acids are methanoic acid ( also known as formic acid) whi...
Organic chemistry is concerned with the compounds of the element carbon. Here we introduce the basic principles of this huge topic.
Concentration Noun :the action or power of focusing all one's attentiona close gathering of people or things.the relative amount of a particular substance contained within a solution or mixture or in a particular volume of space In this topic we will of course be using the third definition of concentration. Students should: 1.34C ...
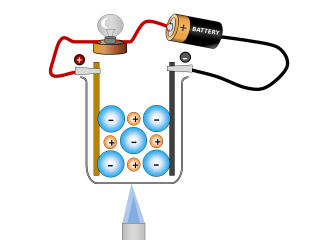
2.23 Activity 3. Aluminium extraction Electrolysis is used to extract reactive metals from their ores. Aluminium is a good example of this. Work through the animation carefully and then answer the questions below: QuestionsAnswers 1. What ions are present in the compound aluminium oxide? 2. What is the aluminium oxide mixed wi...
1.34 understand that ionic compounds have high melting and boiling points because of strong electrostatic forces between oppositely charged ions1.35 understand the relationship between ionic charge and the melting point and boiling point of an ionic compound1.36 describe an ionic crystal as a giant three-dimensional lattice structure held together...
Finding the formula of a compound is important to chemists. We can often predict what the formula might be because we know something about the electron configurations and the way atoms reorganise their electrons to achieve full or empty outer shells. However, here we look at a number of reactions where accurate mass measurement...