The Transition Metals
Colourful compounds
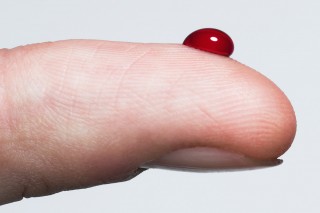
Blood is red, emeralds are green and the Statue of Liberty is ...?
To use this simulation, drag the mouse around to rotate the molecule, and use the scroll wheel to zoom in or out. The iron ions in the haemoglobin are located in the middle of the heme groups. These iron ions are what give heamoglobin, red blood cells, and blood its red colour.
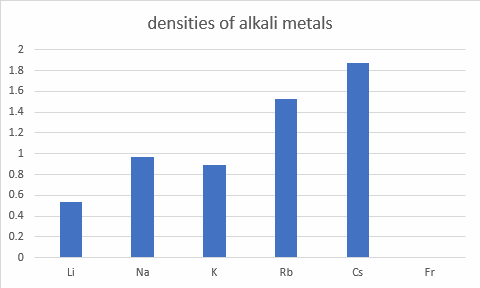
Melting metals
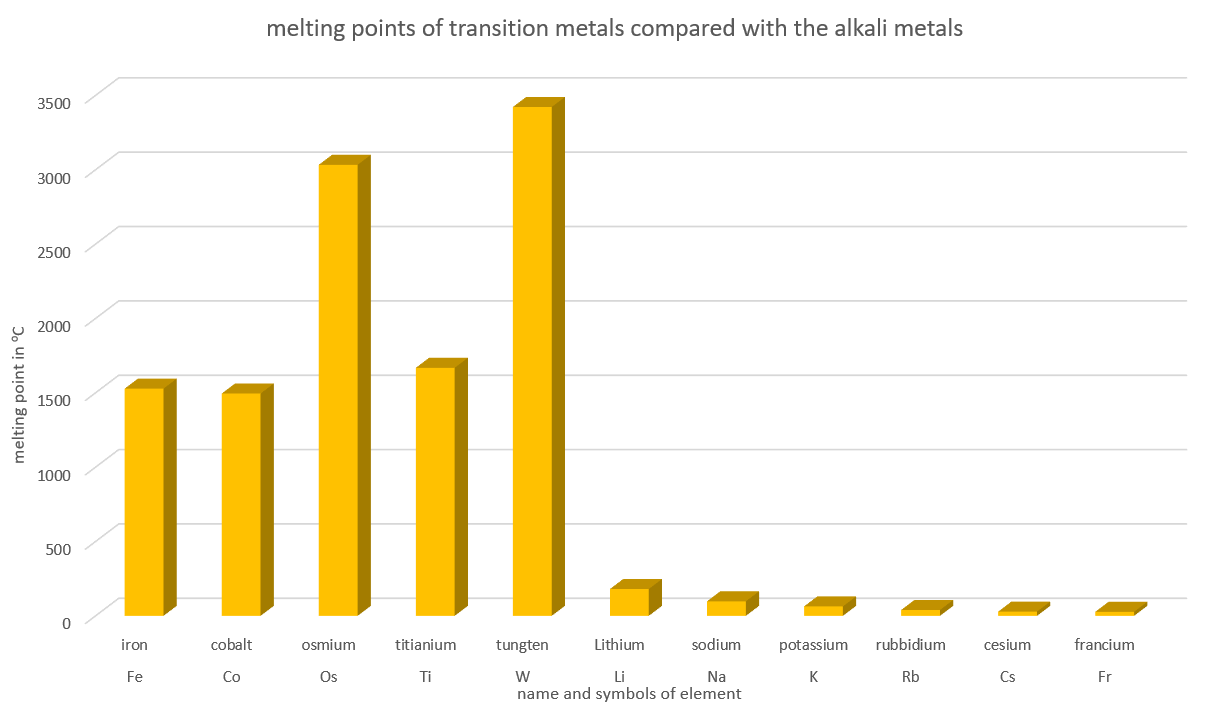
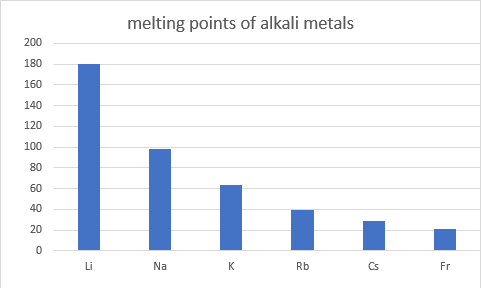
All of the alkali metals appear to have a lower melting point than all of the transition metals ** we have looked at so far. On average, from the data given, then transition metals have an average melting point of 2152oC, And the alkali metals have an average melting point of 72oC.
** except mercury which melts at -41oC
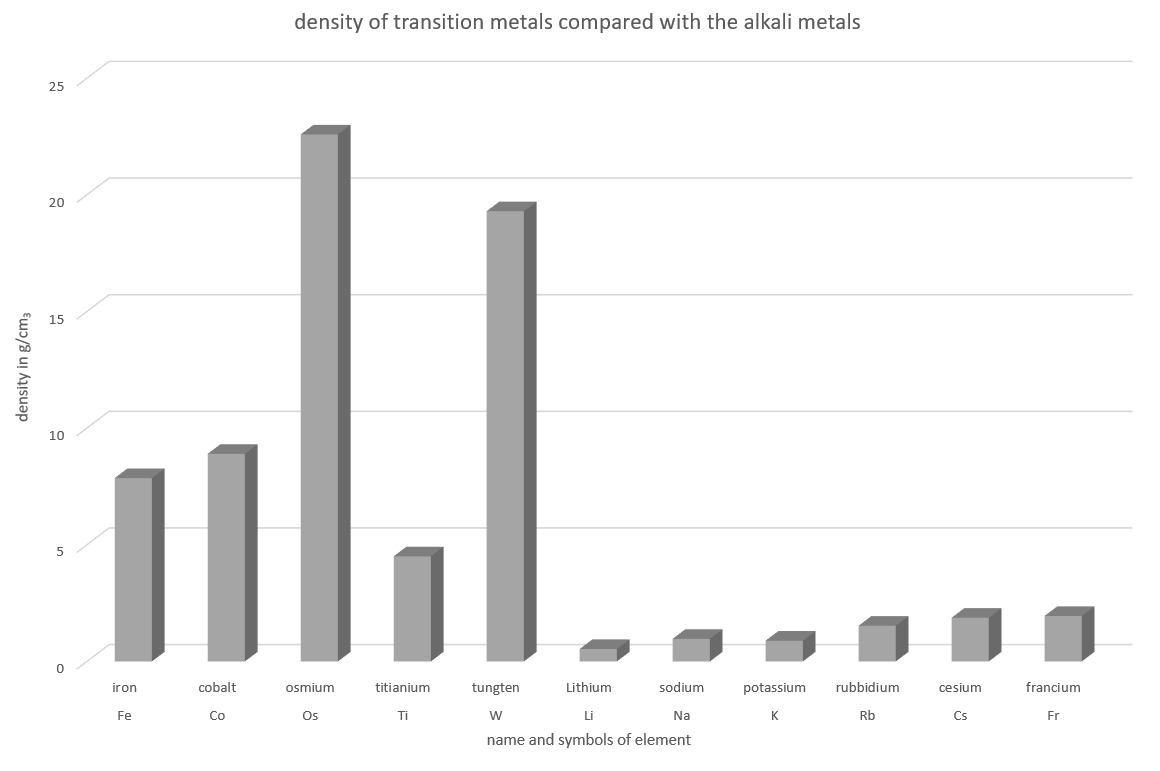
Heavy Metals?
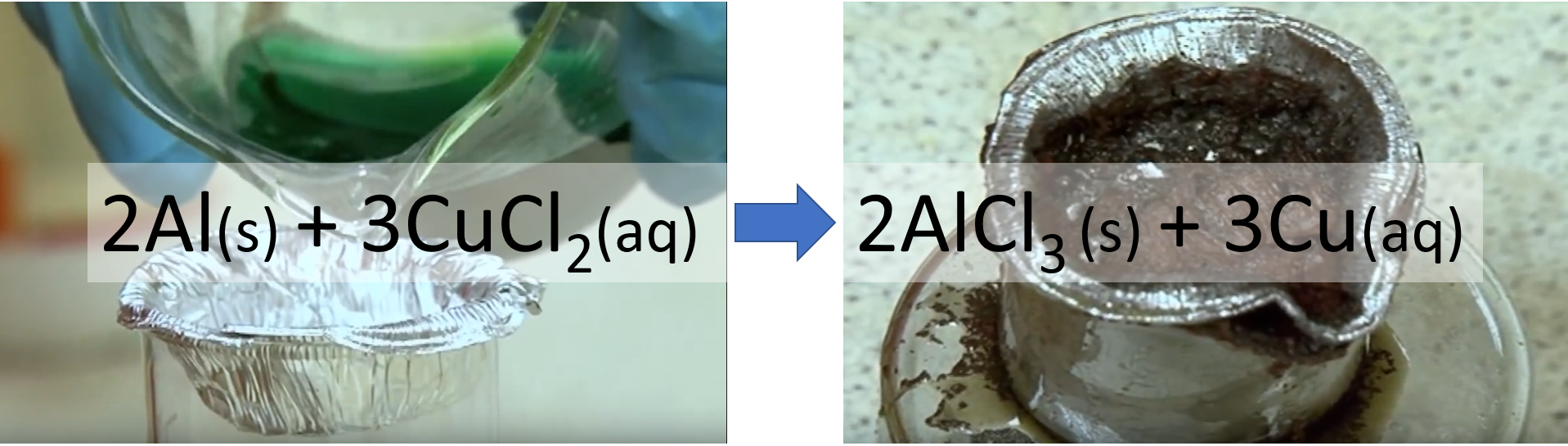
Relative reactivity
Don't try this at home!
What does this reaction tell you about the relative reactivity of copper and aluminium.
What does the video of the reaction tell you about the energy change involved?
aluminum is more reactive than copper, therefore gives away its electrons. Copper ions are turned into copper atoms, and the aluminum atoms are turned into ions.

The Reactivity Series
lead trees, furry worms, and silver ferns
Enter your text here ...
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.