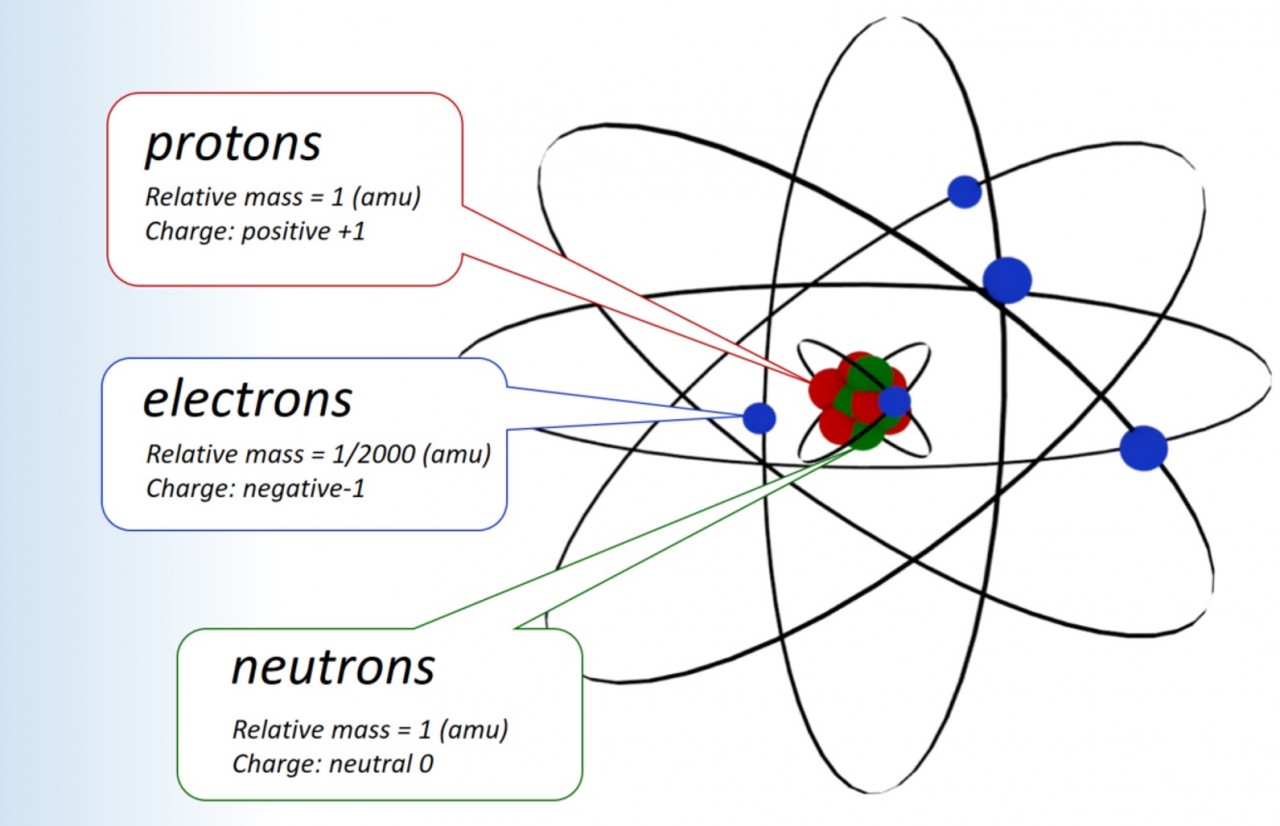
1.15 Sub atomic particles Students should: 1.15 know the structure of an atom in terms of the positions, relative masses and relativecharges of sub-atomic particles. Here we look at the particles inside the atom. Sub atomic particles are the particles found inside atoms. These include protons, neutrons and electrons: protons a...
Relative masses allow chemists to "count out" atoms and molecules so they can ensure that the appropriate amounts of substances are reacted.
Oxygen is a key component of the atmosphere and is critical for the support of life. Its reactivity makes it essential for respiration and combustion reactions - both of which release energy. Oxides are formed when oxygen combines with other elements. Much of Earth's crust (48.5% by mass) is composed of oxygen in the form of oxides ...
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds. 1.46 Activity 2. Crossing and dotting Students should: 1.46 understand how to use dot-and-cross diagrams to represent covalent bonds in molecules. Dot and cross diagrams are a useful way of representing how atoms share electro...
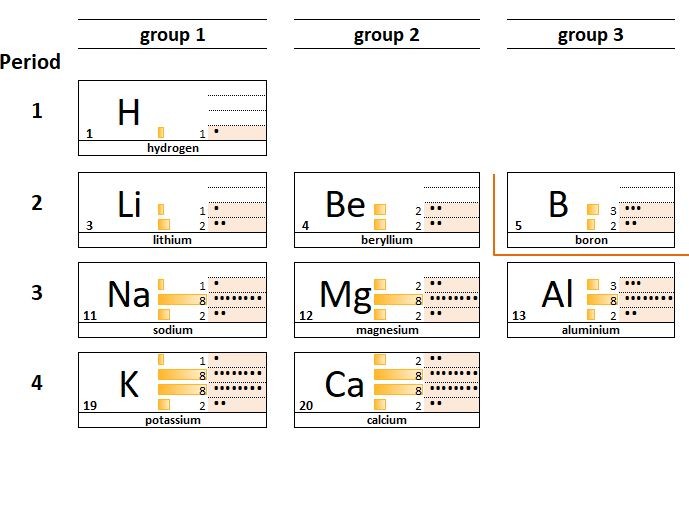
1.23 Family resemblances Hydrogen is often listed above group 1 the Alkali metals. Although like group 1 atoms, hydrogen atoms have a single electron in their outer shell, Hydrogen is not a metal. Students should: 1.23 understand why elements in the same group of the Periodic Table have similarchemical properties 1.24 und...
1.55 Using electrolysis Electrolysis happens when an electric current is passed through an ionic compound which has been melted or dissolved. Electrolysis has a wide range of uses including : electroplating, extraction of aluminium, production of chlorine and the purification of copper . This post helps you to unders...
1.3 Activity 4. A particular problem Students should: 1.3 understand how the results of experiments involving the dilution of coloured solutions and diffusion of gases can be explained. Particle theory states that all matter consists of many, very small particles which are constantly moving or in a continual state of motion. T...
Many chemical reactions can be reversed. When this happens we can describe a forward reaction and a back reaction. Sometimes the forward and back reaction take place at equal rates. When this happens an Equilibrium is established Students should: 3.17 know that some reactions are reversible and this is indicated by the symbo...
Modern methods Activity 1 : Phytomining and Bioleaching Watch the video carefully, stopping where necessary to answer the questions below: QuestionsAnswers Explain the main difference between high grade ore and low grade ore.What is happening to the supply of high grade copper ore?Explain what is meant by bioleachin...
Background This topic is mostly about equilibria so it is important for students to understand that concept first. Before tackling this topic make sure you : understand dynamic equilibriacan write and manipulate equilibrium expressions for a range of reactions.can estimate the pH value of a solution using universal indicator know th...