When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds.
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds. 1.45 Activity. Opposites attract Students should: 1.44 know that a covalent bond is formed between atoms by the sharing of a pair ofelectrons1.45 understand covalent bonds in terms of electrostatic attractions Play the video t...
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds.
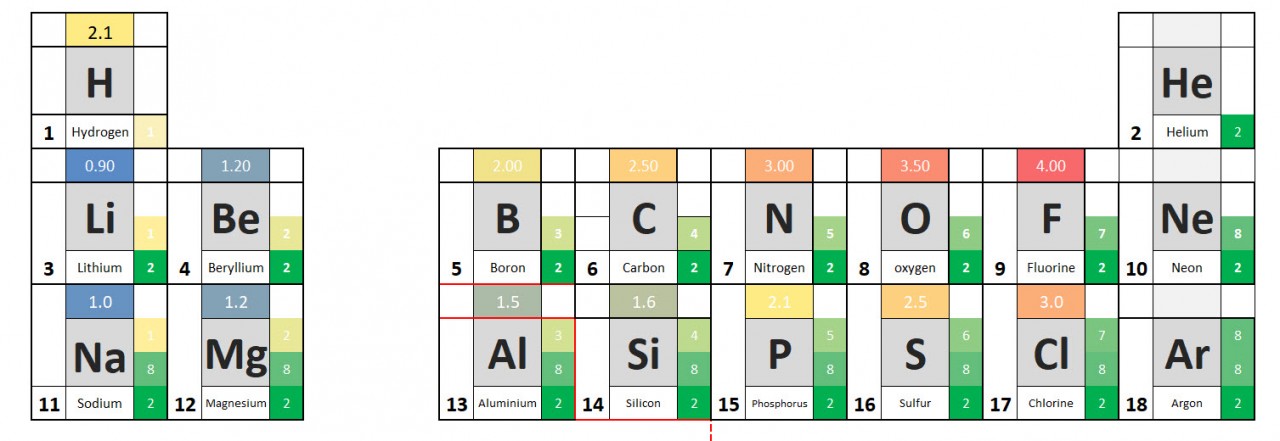
1.38 Activity. Know your ions. Students should: 1.38 know the charges of common ions listed You will have noted from the video that metal atoms tend to lose the electrons in their outermost electrons and form positive ions. The charge of the ion formed is equal to the number of electrons lost. Non metal atoms gain el...
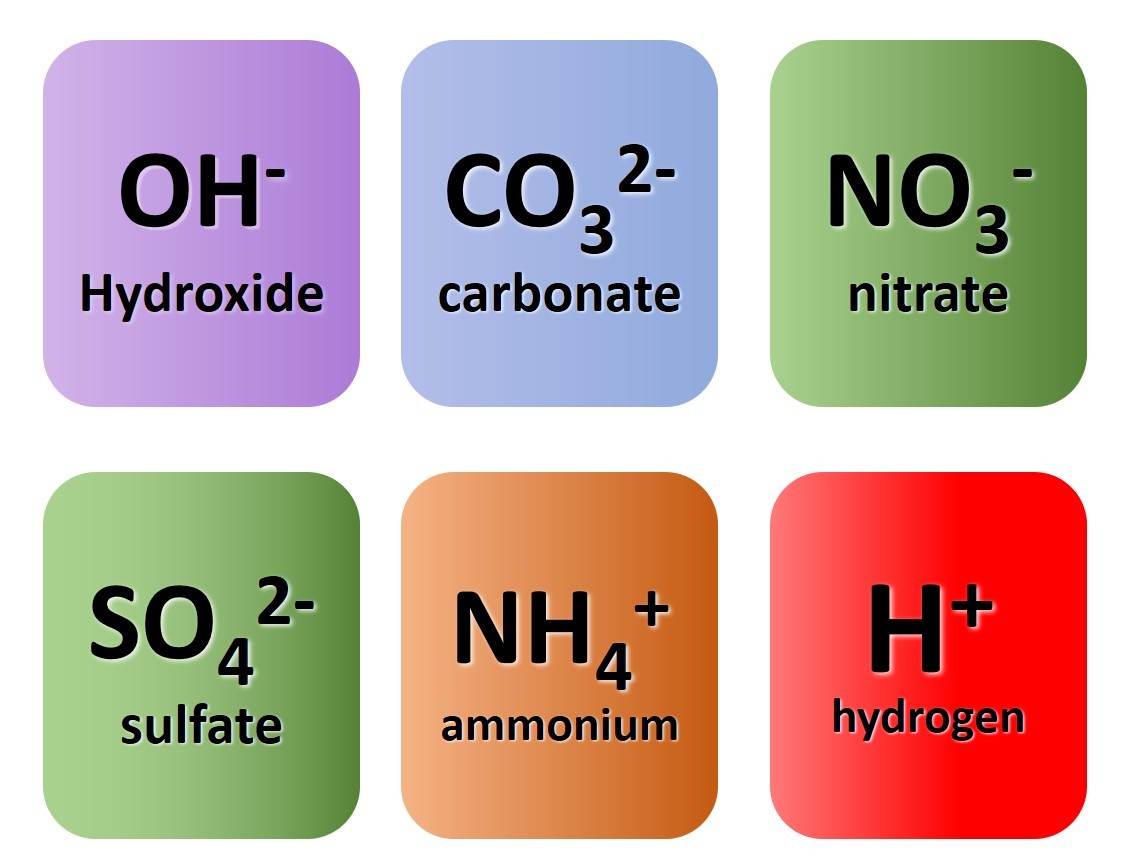
1.39 Activity. Finding formulae Students should: 1.39 write formulae for compounds formed between the ions listed Watch the video closely pausing where instructed. Use the information given to work out the correct formulae for the following compounds: Name of compoundFormula lithium nitridepotassium oxidezinc oxidesilver chlorideLi3N&nb...
Ionic compounds form when the atoms of a metal combine with the atoms of a non - metal
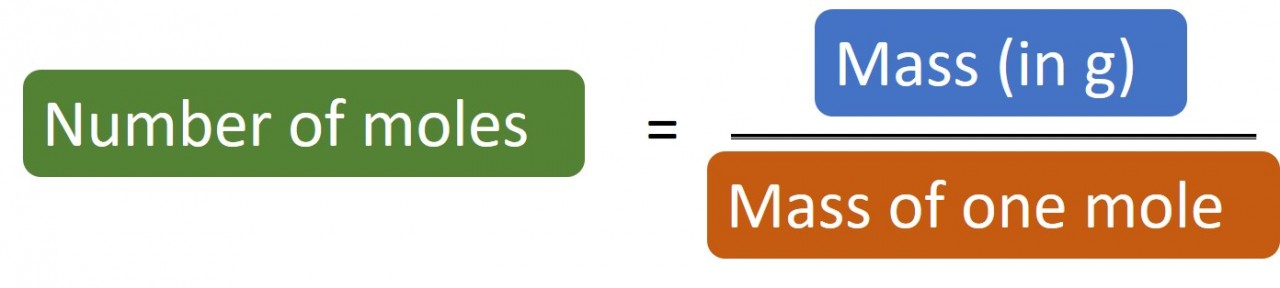
1.27 What is a mole? Students should: 1.27 know that the mole (mol) is the unit for the amount of a substance 1.28 understand how to carry out calculations involving amount of substance, relativeatomic mass (Ar) and relative formula mass (Mr) A mole (mol) is the quantity of anything that has the same number of particles as there are ato...
1.28 Calculating moles The following masses of elements all contain one mole of atoms: 12.0 g Carbon, 32.1 g Sulphur, 14 g Nitrogen, 24.3 g Magnesium. So if you have 24 g carbon you have 24/12 = 2 moles carbon atoms. Likewise if you have 1.4 g nitrogen you have 1.4/14 = 0.1 moles nitrogen atoms. Rearrangin...
Relative masses allow chemists to "count out" atoms and molecules so they can ensure that the appropriate amounts of substances are reacted.
The elements in the periodic table are organised in order of increasing atomic number.