Physically gases have a number of similarities. For example - all gases will: fill the container in which they are placed and adopt its shapeexpand when heated and contract when cooledbe compressed if subjected to increased pressureHowever despite these physical similarities many gases are chemically very different from one another....
Many organic molecules can be made to join up with one another to form very long molecules which can be thousands of atoms long. This makes new materials with water resistant properties. Many of these materials will soften when heated and can therefore be easily moulded into complex shape.These materials are known are...
2.34 Defining salts Common "table" salt ( sodium chloride) is found dissolved in large quantities in seawater. Sodium Chloride is just one example of the many compounds which can be called salts. Most salts are crystalline ionic compounds A salt is defined as : A compound resulting from a chemical reaction of an acid...
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds. 1.44 - 1.45 Activity. Opposites attract Students should: 1.44 know that a covalent bond is formed between atoms by the sharing of a pair ofelectrons1.45 understand covalent bonds in terms of electrostatic attractions Play the ...
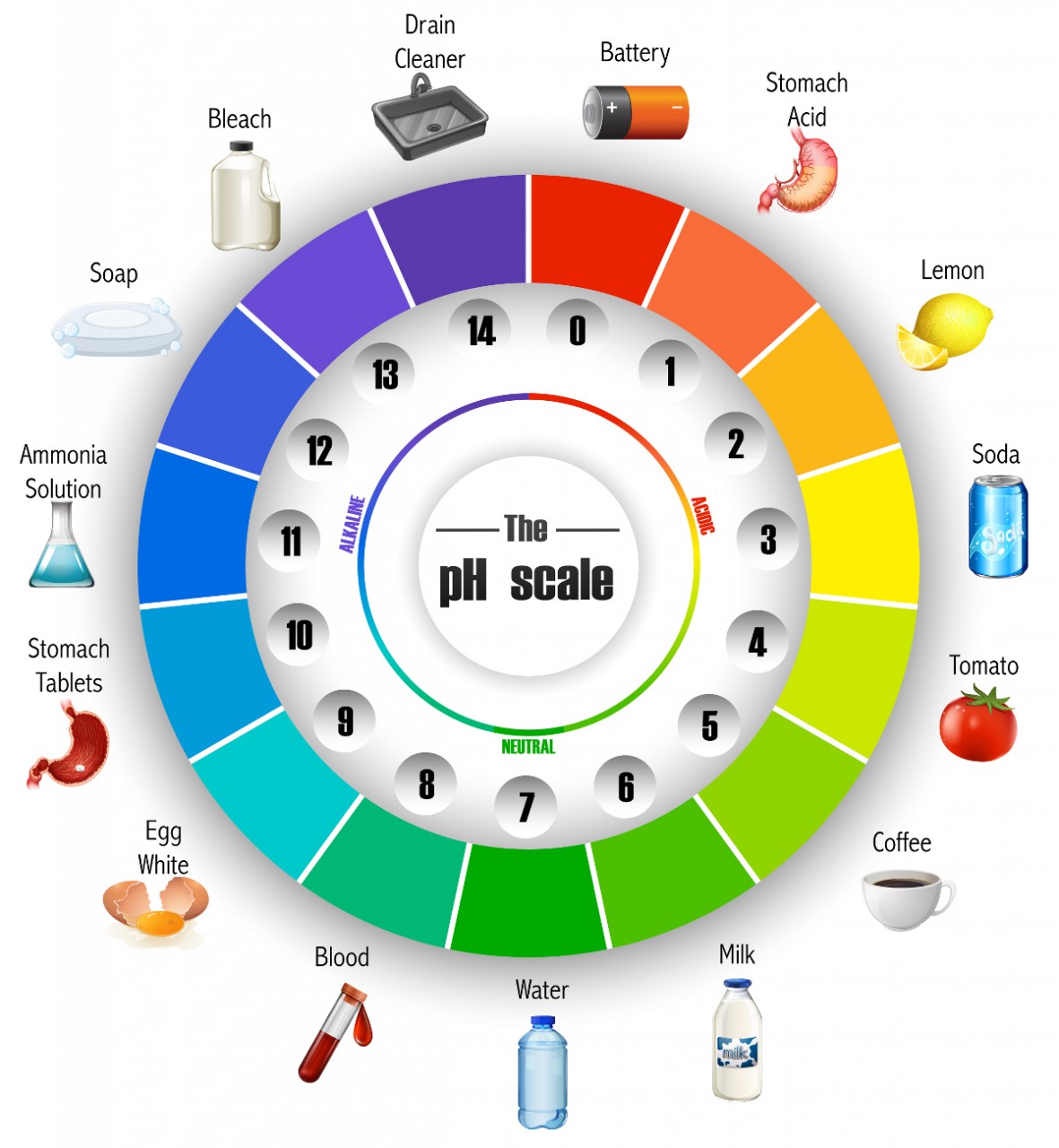
Acids and alkalis are all around us; many everyday foods, drinks, cleaning products are acidic or alkaline. Study the image of the pH scale "wheel". This scale gives a measure of the acidity or alkalinity of a substance. A value of pH 7 is regarded as neutral . Substances with a pH value greater than 7 are regarded as alkaline ...
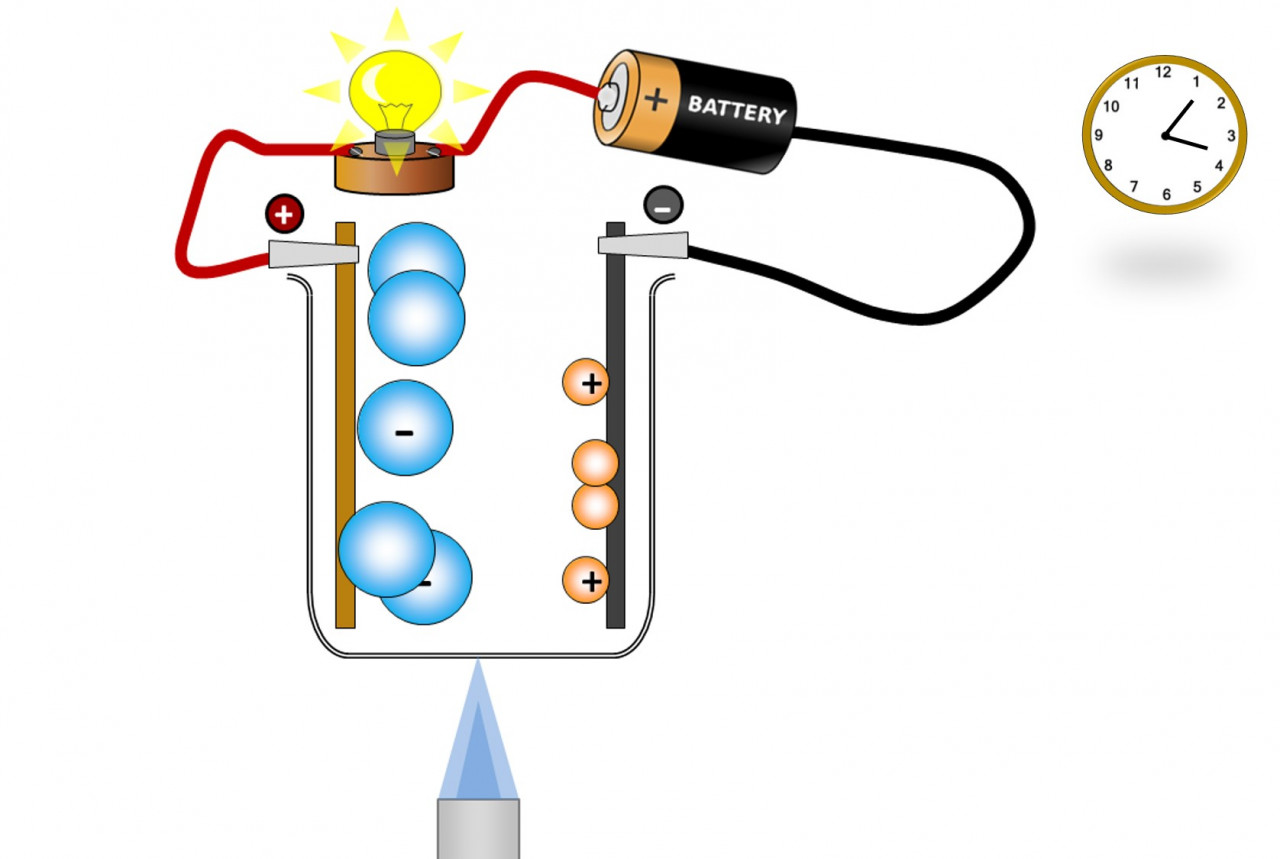
Here we investigate electrolysis and try to explain our findings with a few diagrams and animations. In this diagram we are showing how heating causes the ions to become mobile, and then the electric field causes the ions to separate. This allows us to separate elements in a molten ionic compound. Electrolysing molten compounds: I...
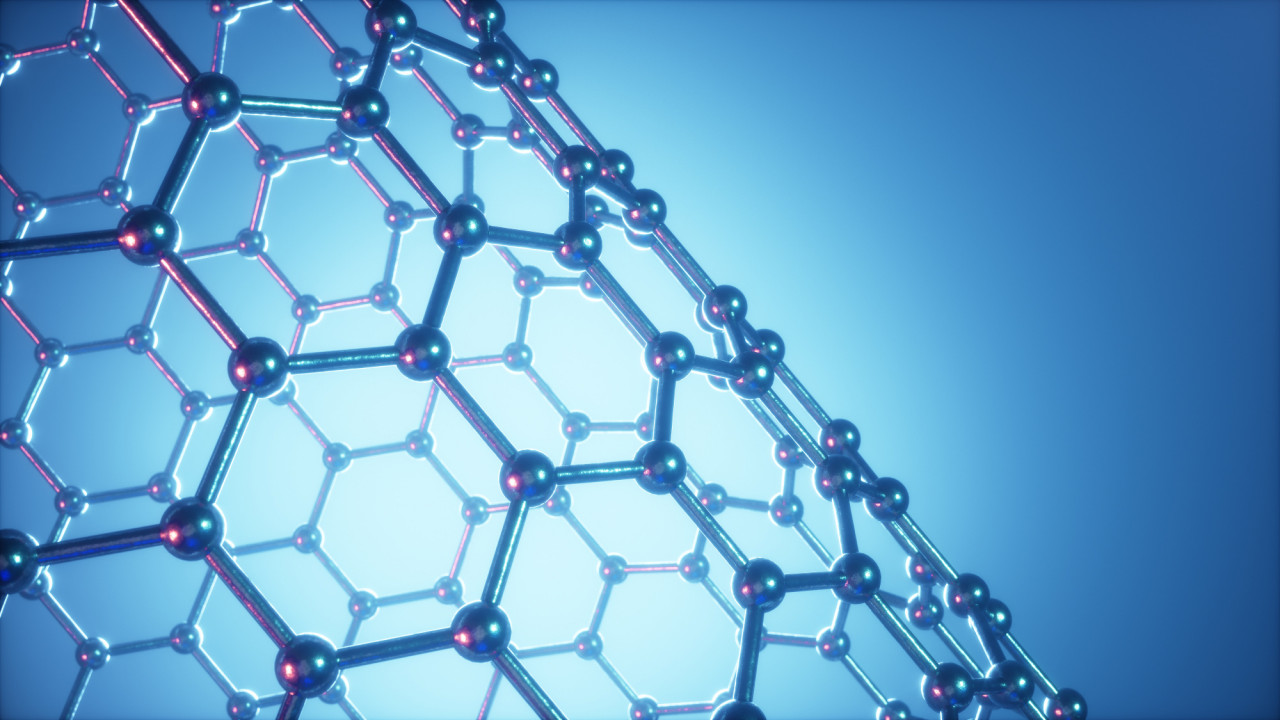
Covalent bonds form when electrons are shared between nuclei Atoms which can form two or more covalent bonds can bond with other similar atoms or themselves and form covalent lattices. Carbon in the form of diamond is a good example, as is silicon dioxide in the form of quartz. A valuable lattice Activity. Diamond - clos...
Look at the images of ice and diamond. In some senses you might say the two substances are similar. Both substances are solid, transparent and crystalline. In terms of bonding, they have similarities too : In ice, atoms of oxygen are held to hydrogen atoms by covalent bonds. In diamond atoms of carbon ar...
Look at the images of ice and diamond. In some senses you might say the two substances are similar. Both substances are solid, transparent and crystalline. In terms of bonding, they have similarities too : In ice, atoms of oxygen are held to hydrogen atoms by covalent bonds. In diamond atoms of carbon ar...
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds.