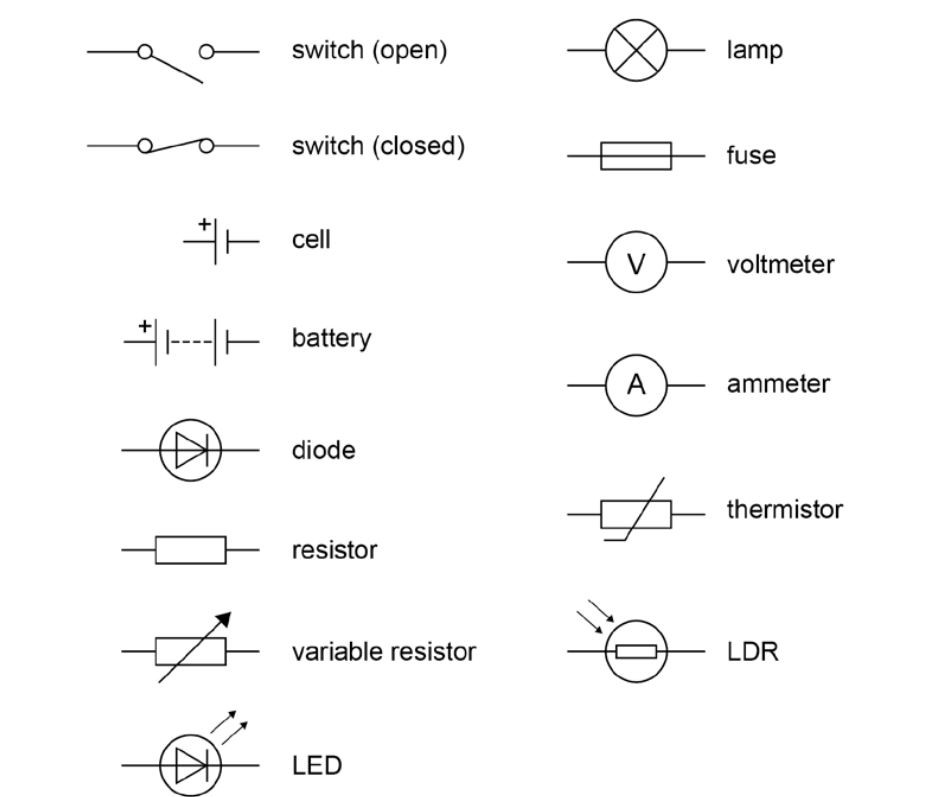
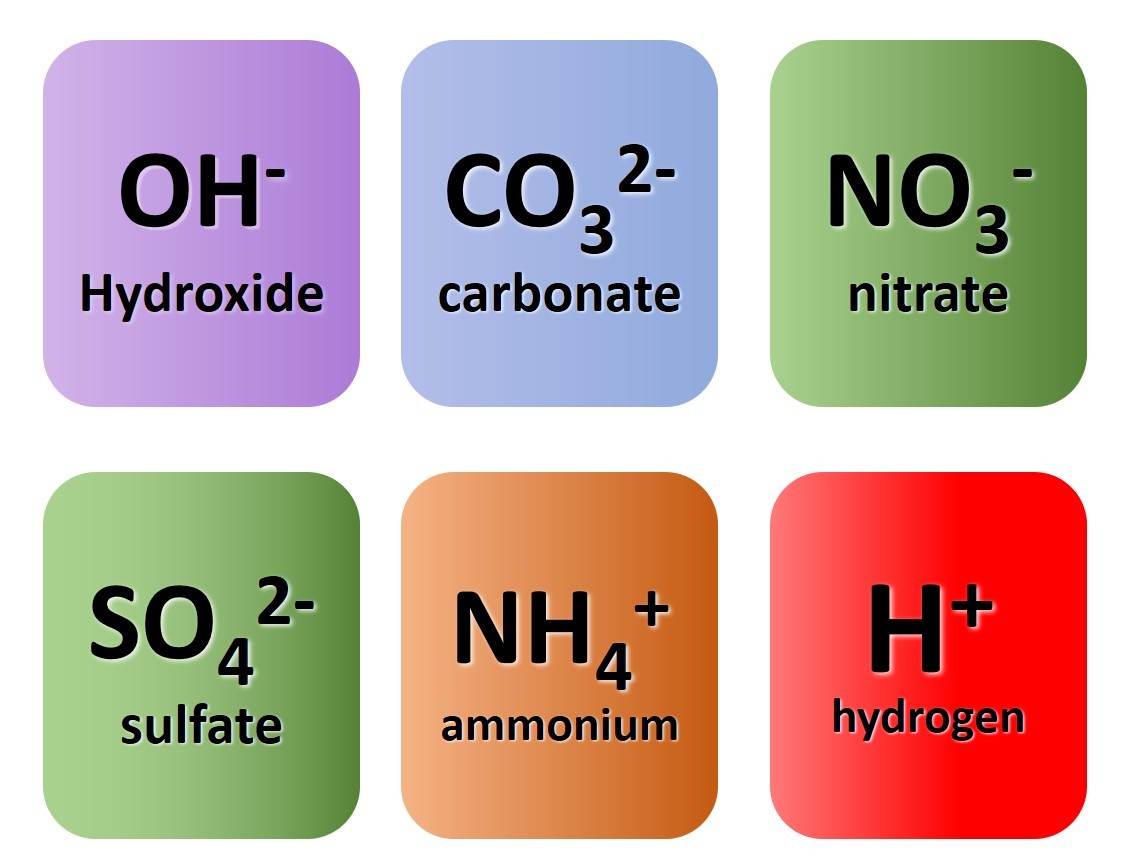
the formulae of five polyatomic ions and one monatomic ion to be learnt.
1.39 Activity. Finding formulae Studen...
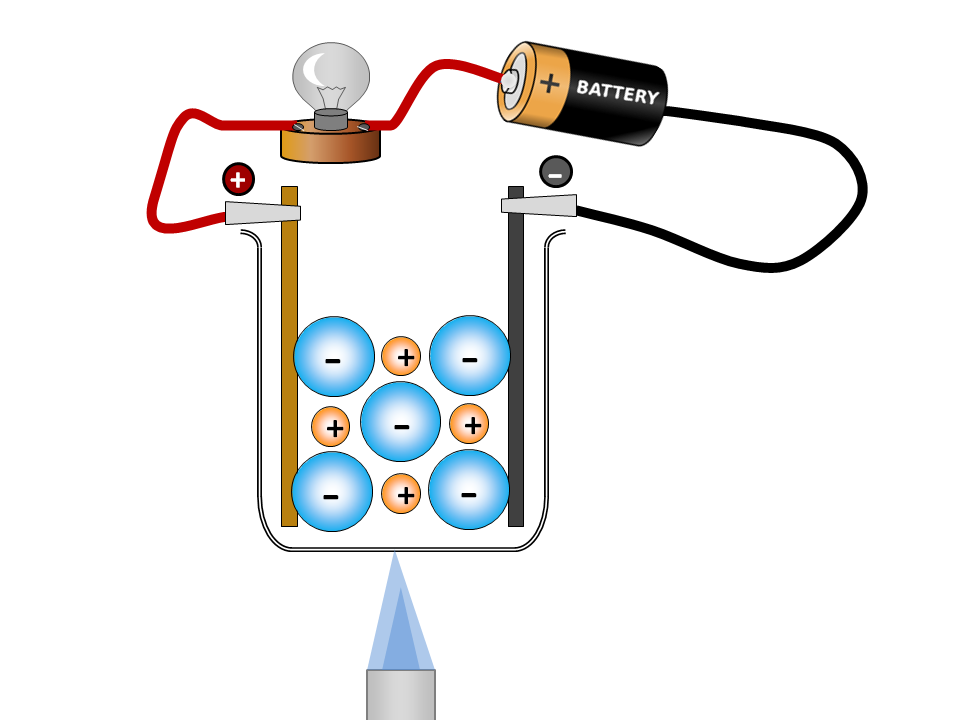
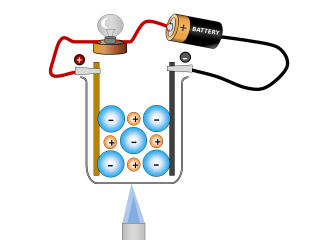
Rust forms on iron and steel as a result of a redox reaction between iron and oxygen.
2.15 - 2.20 Loss and gain Oxidation and redu...
Intermolecular forces are responsible for holding together the two strands of a DNA molecule
Before tackling this topic make sure you are&...
water expands when it freezes and ice therefore floats
1. Why is water a liquid and not a gas (...
An Exothermic reaction. Acetylene (Ethyne) releases a lot of energy when it burns.
Enter your text here ... Introduction Every chemi...
If a nucleus was the size of a raisin, the rest of the atom would be the size of a sports stadium
If a nucleus was the size of a raisin, the rest of the atom would be the size of a sports stadium
Atomic models Atoms are the building blocks ...
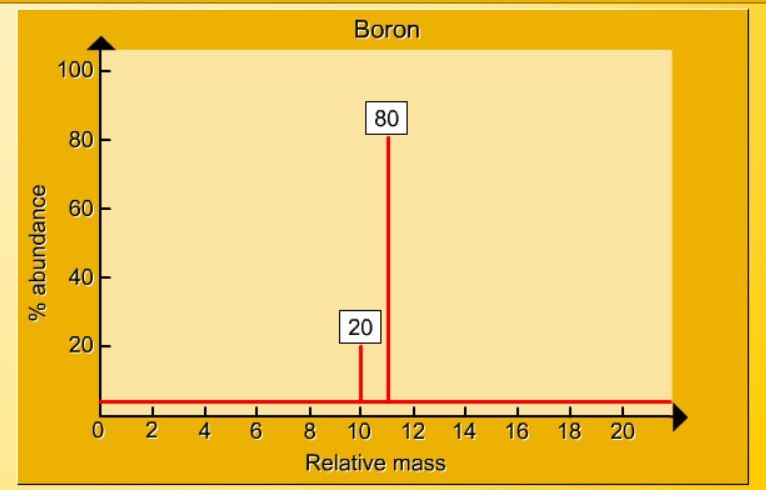
The mass spectrum of boron
1.17 Activity 2. Calculating relative atomic...
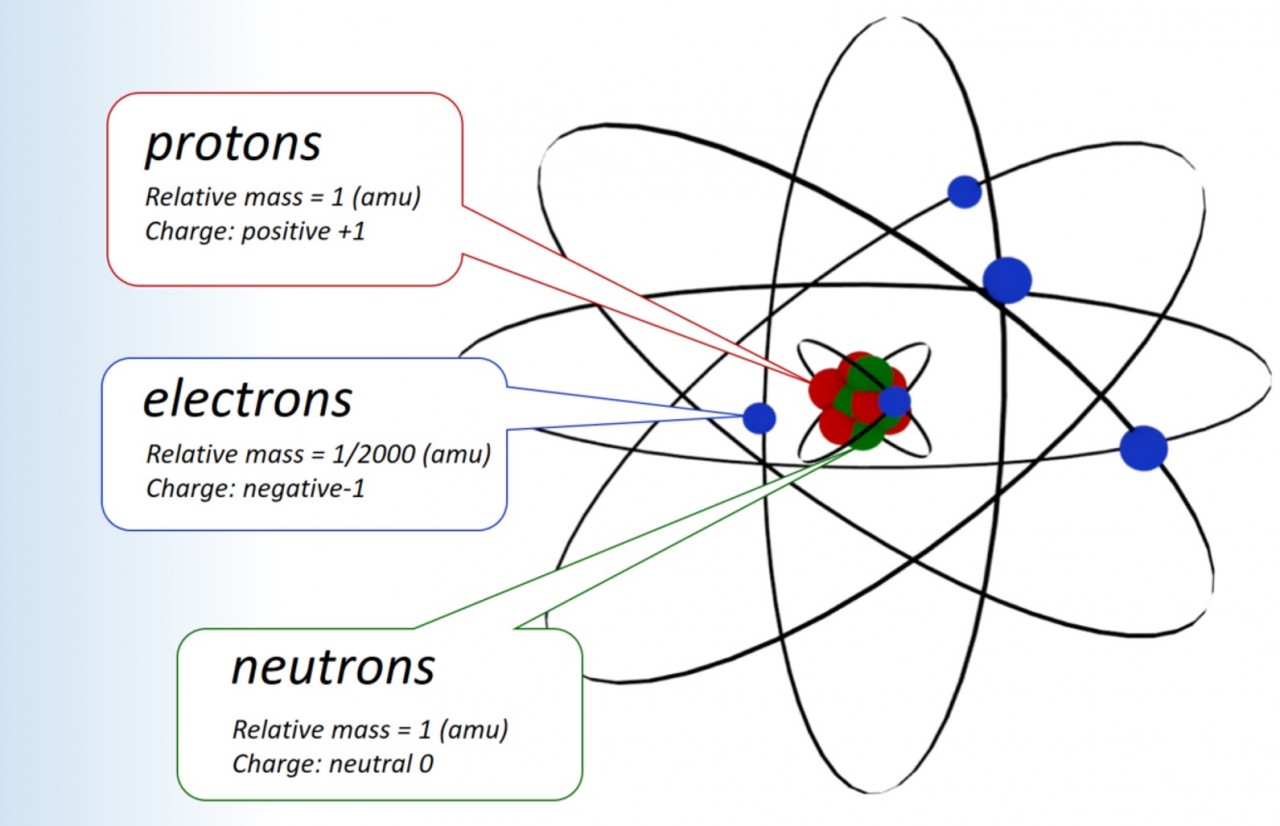
masses and charges of subatomic particles
1.15 Sub atomic particles Students sho...