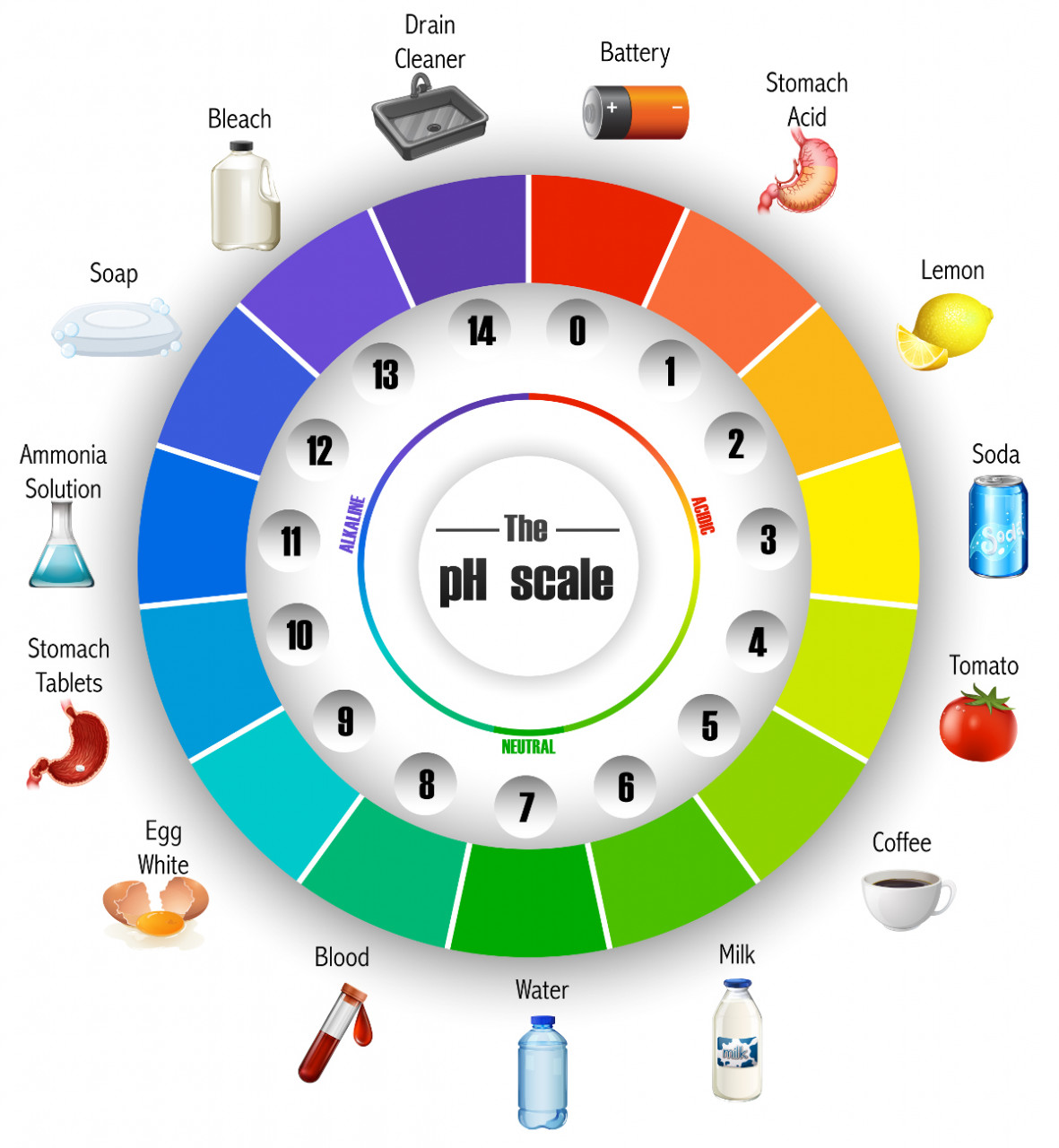
Universal indicator is useful for giving an estimated value for the pH of a substance
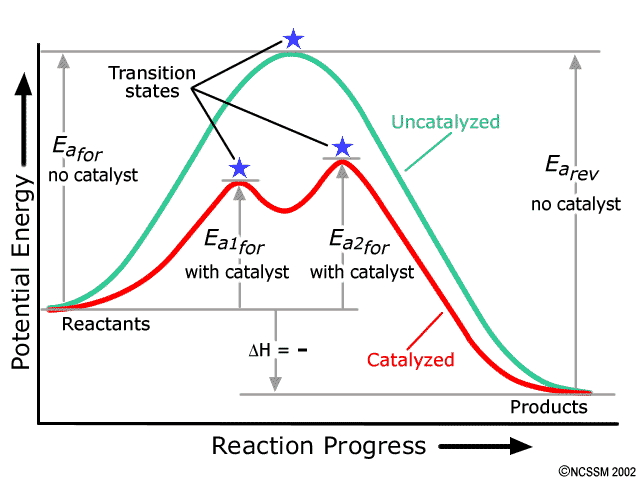
This is one of the tougher topics in year 13 chemi...
Quinoa is a complete protein, meaning that it contains all nine essential amino acids that our bodies cannot make on their own.
EdexcelOCRAQA Enter your text here ... Enter your ...