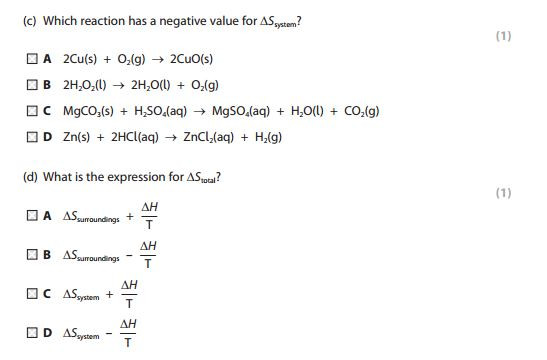Entropy
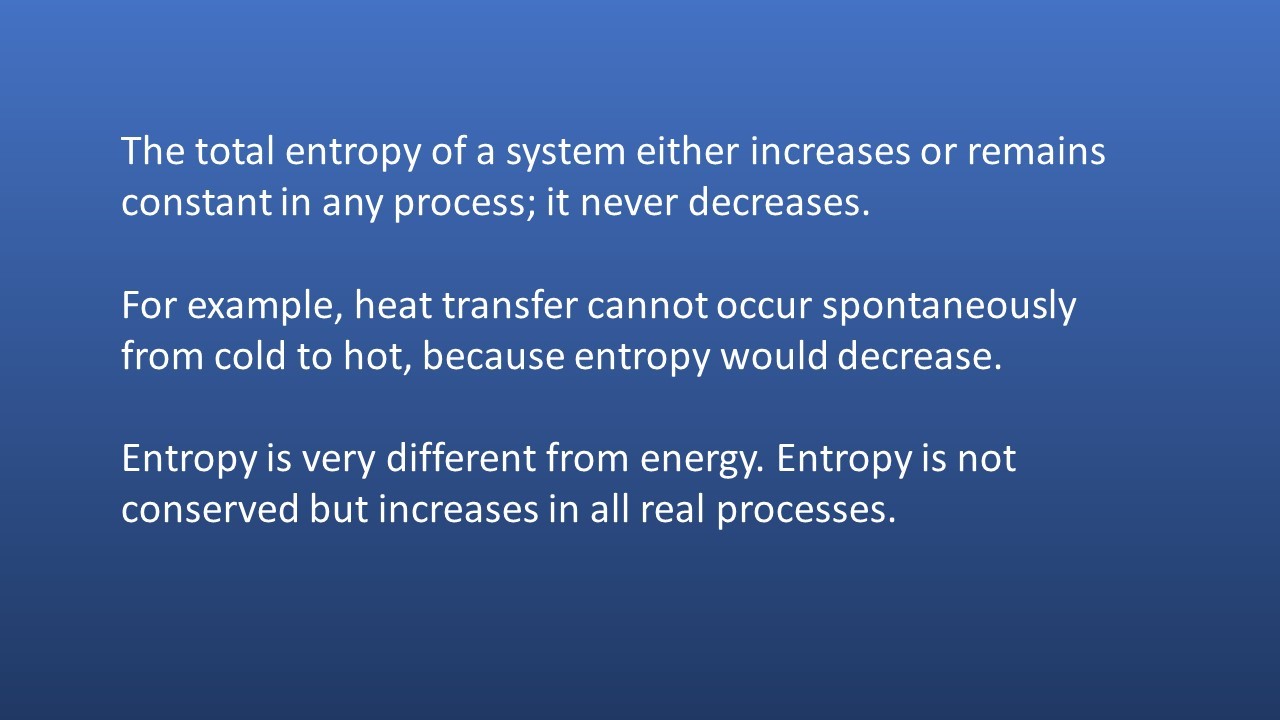
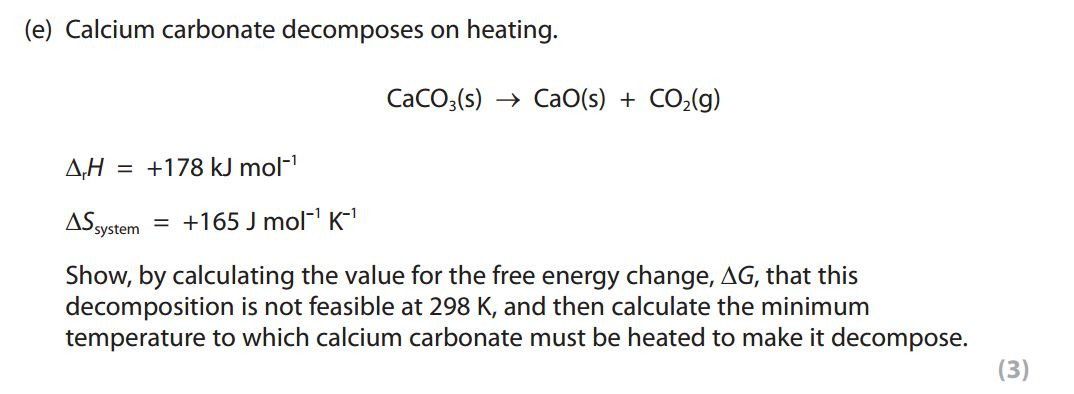
Exothermic reactions always lead to an increase in entropy of the surroundings. In an example like this - the burning of magnesium in oxygen - the entropy of the system decreases because a giant ionic lattice ( Magnesium Oxide) is formed from the magnesium metal and oxygen (gas).