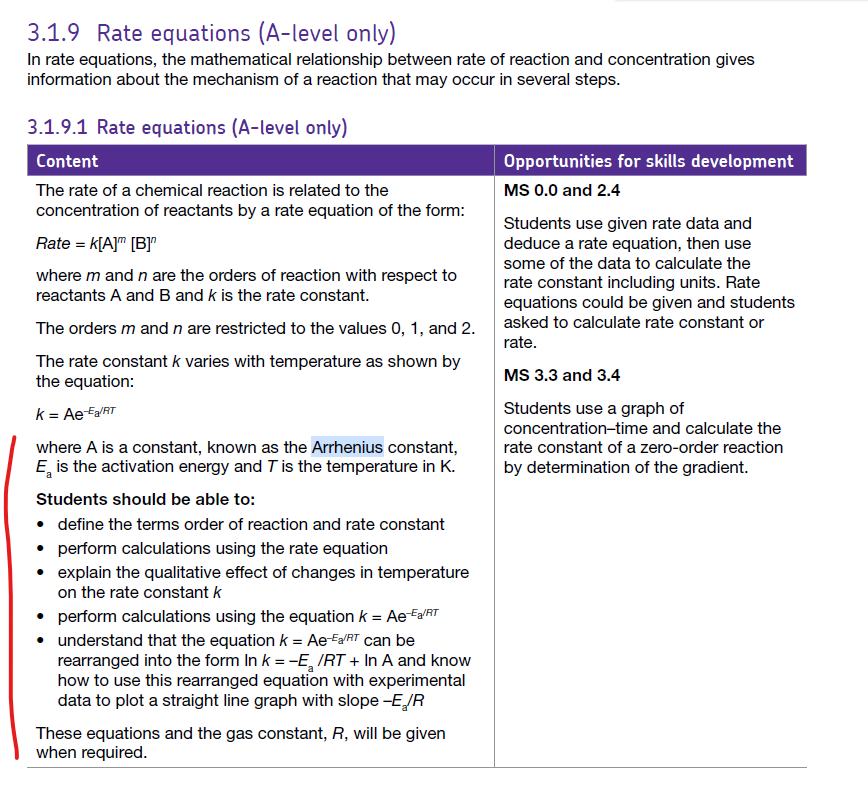
OCR Learning outcomes Additional guidance Synthesis and separation. Purification methods. Recrystallisation is a purification technique used to separate a solid compound from impurities by exploiting differences in solubility. How it works Dissolve the impure solid in a hot solvent in which the desired compound is highly soluble at high tempe...
Introduction Group 17 ( used to be group 7) contains a set of non-metals known collectively as The Halogens. Like many families, the members of Halogen family have a number of similarities to each other. However, they also have some very important differences. Activity 1: Ferocious Fluorine This video shows&nbs...
3.9 - 3.16 Rates of reaction paint must not dry too quickly 3.9 - 3.16 What is rate? How fast a chemical... https://www.mychem.co.uk/index.php/igcse-chemistry/physical-chemistry/rates-of-reaction Any measurable factor which changes as a reaction proceeds can be used to measure the rate. The gradient of graphs give a reading for the reac...
Intro image Edexcel 1Edexcel 2OCR Enter your text here ... Exothermic reactions always lead to an increase in entropy of the surroundings. In an example like this - the burning of magnesium in oxygen - the entropy of the system decreases because a giant ionic lattice ( Magnesium Oxide) is formed from the magnesium metal...
Hot and quick Investigating rates Results The rate of a chemical reaction is significantly affected by its temperature. the effect of temperature on rate can be shown visually using the Maxwell - Boltzmann distribution. OCR Enter your text here ... Edexcel AQA The Maxwell Boltzmann curve is a distribution curve which show...
Background This topic is mostly about equilibria so it is important for students to understand that concept first. Before tackling this topic make sure you : understand dynamic equilibriacan write and manipulate equilibrium expressions for a range of reactions.can estimate the pH value of a solution using universal indicator know th...
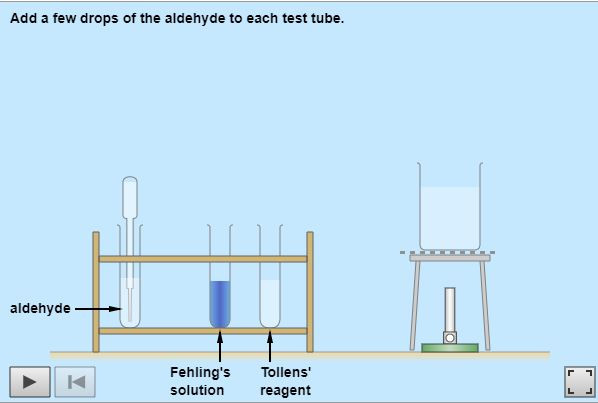
Carbonyl compounds all contain a C=O double bond. This is usually formed when an alcohol is oxidised using an oxidizing agent such as acidified sodium dichromate. The position of the C=O bond determines whether the compound is an aldehyde or ketone OCREDEXCEL Reactions of carbonyl compounds Enter your text here...
EdexcelOCRAQA Production of alcohols Enter your text here ... Oxidation of alcohols Enter your text here ... Elimination from alcohols With phosphorus pentachlorideMaking HBrIodo alkanes Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your text here ... Enter your te...
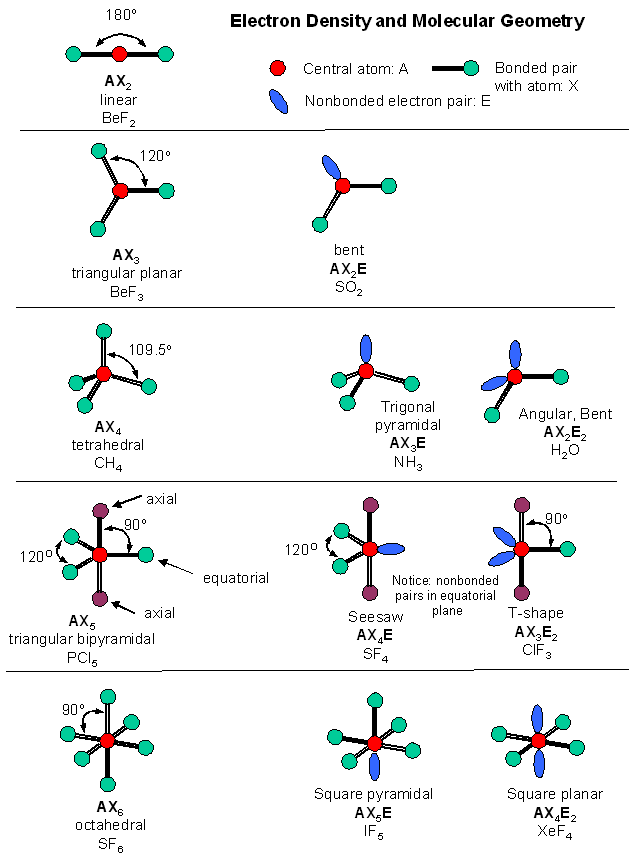
Accounting for electrons. The images in this slideshow here show how we can account for the way in which electrons are shared between atoms. Use the first of the three buttons to the top left of the animation to see the outer electron configuration of each atom. Construct a table to show the name of the molecule, formula, no.of bond...
1. Why is water a liquid and not a gas ( between 0 and 100oC) ? Water has a Relative Molecular mass ( Mr) of 18 ... Oxygen's Mr is 32. If Mr was the only factor that affects the state of a substance then wouldn't you expect water to be a gas ? 2. Why does Ice float on water? Most liquids become more dense as they cool. This caus...