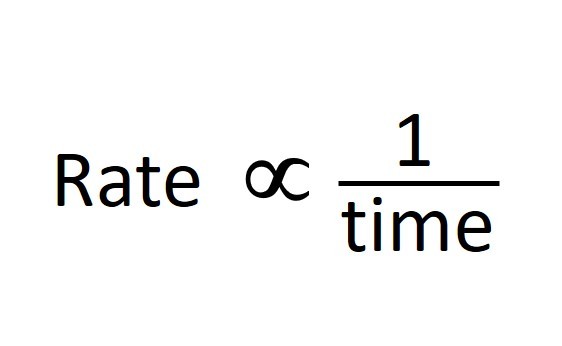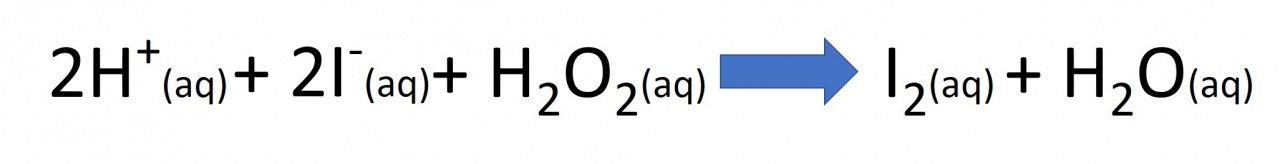Methods of following reactions

3.9 - 3.16 Rates of reaction
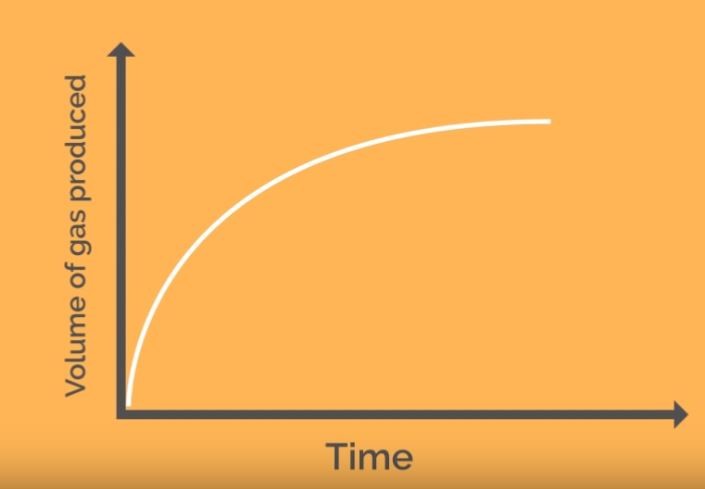
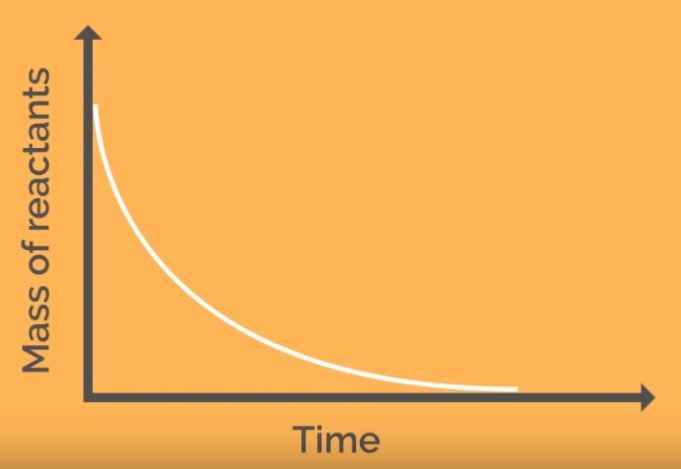
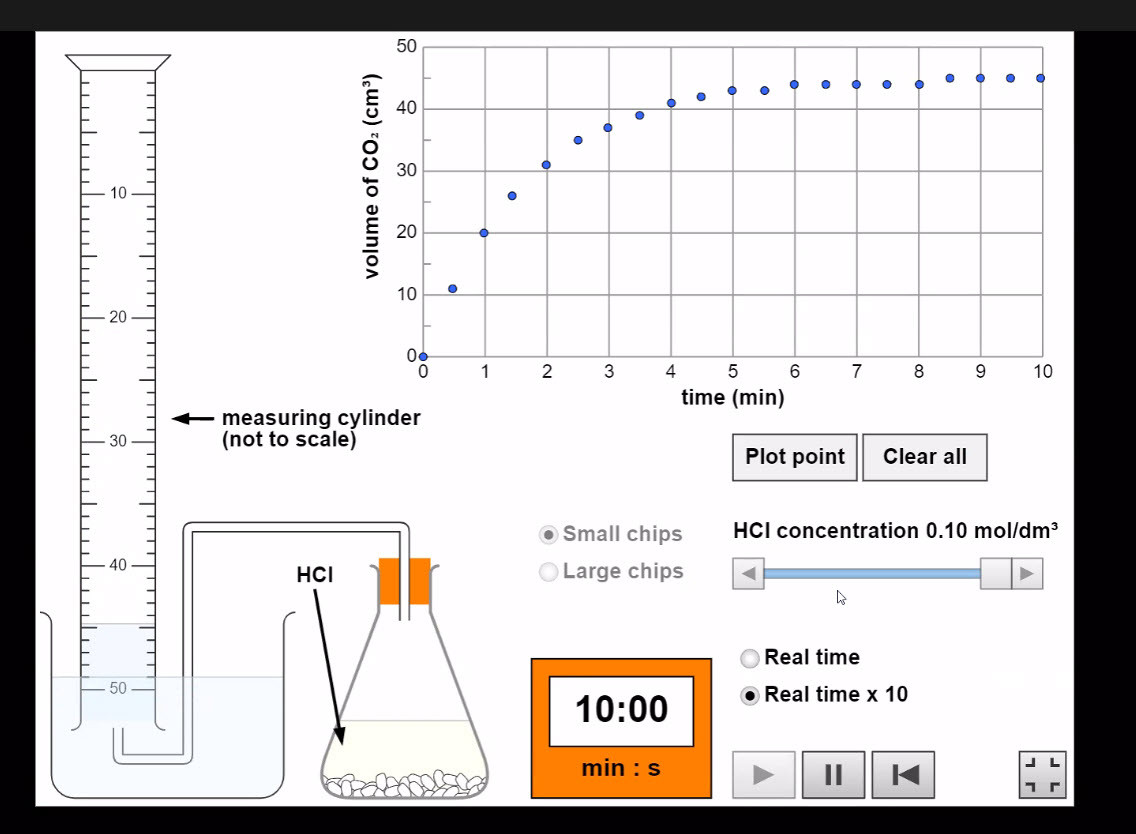
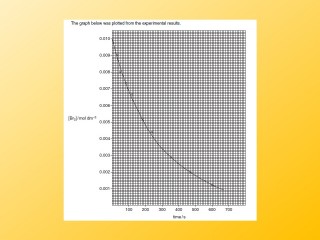
Any measurable factor which changes as a reaction proceeds can be used to measure the rate. The gradient of graphs give a reading for the reaction rate.
Dodgy data discussion
This classic reaction can be used in the laboratory to produce either of the two graphs above.
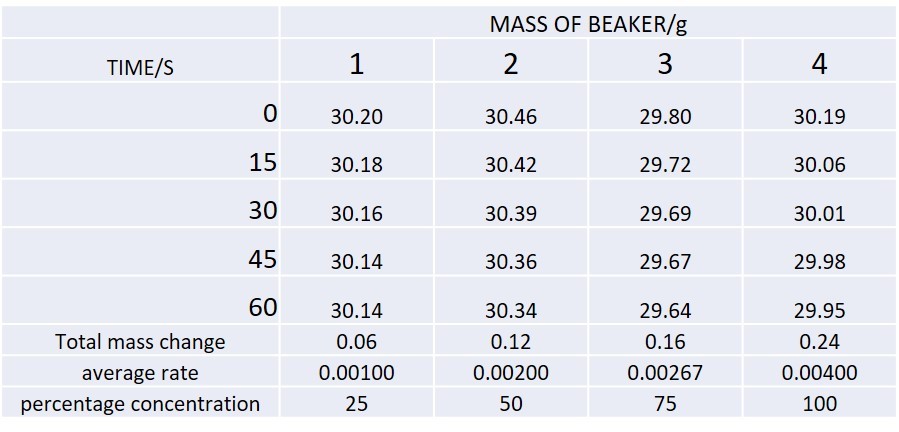
You could measure the mass of the reaction mixture vs time. This will give data as shown in the table. Looking at the data what can you say about how the rate of reaction changes as the reaction proceeds?
Stop the clock
The iodine clock reaction is a classic reaction which can be used to compare the initial rate of a reaction.There are two reactions taking place simultaneously in the solution. Both are redox reactions:
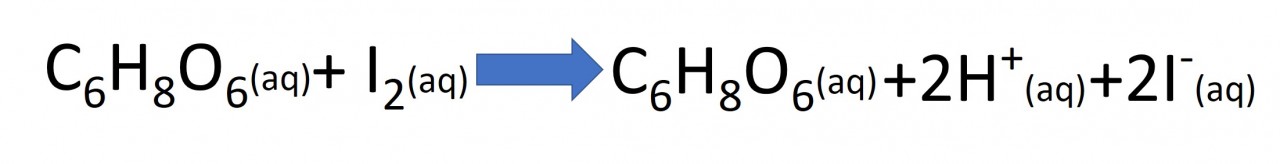
But iodine produced in the above reaction is immediately removed by the vitamin C.Now after a short time as the reactions keep proceeding in this fashion, the Vitamin C gets gradually used up. The Vitamin C creates a clock reaction (1 - 2 minutes) and once it is used up, the solution turns blue, because now the iodine element and starch are present.
Experiment simulation: Calcium Carbonate and hydrochloric acid
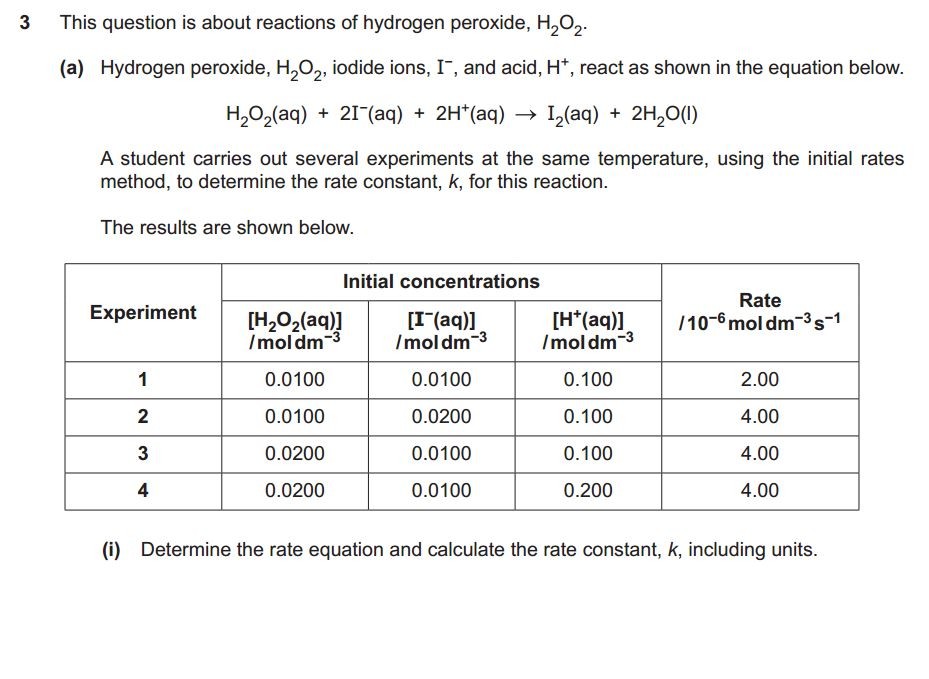
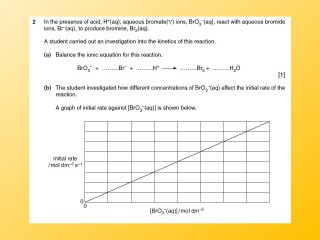
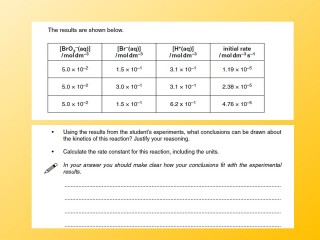
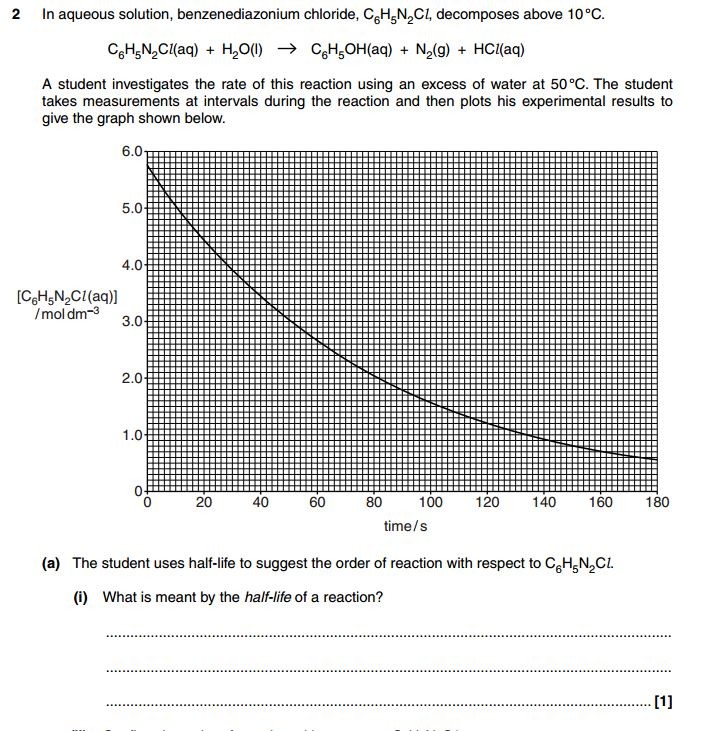
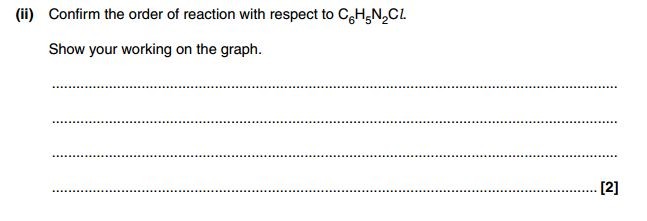
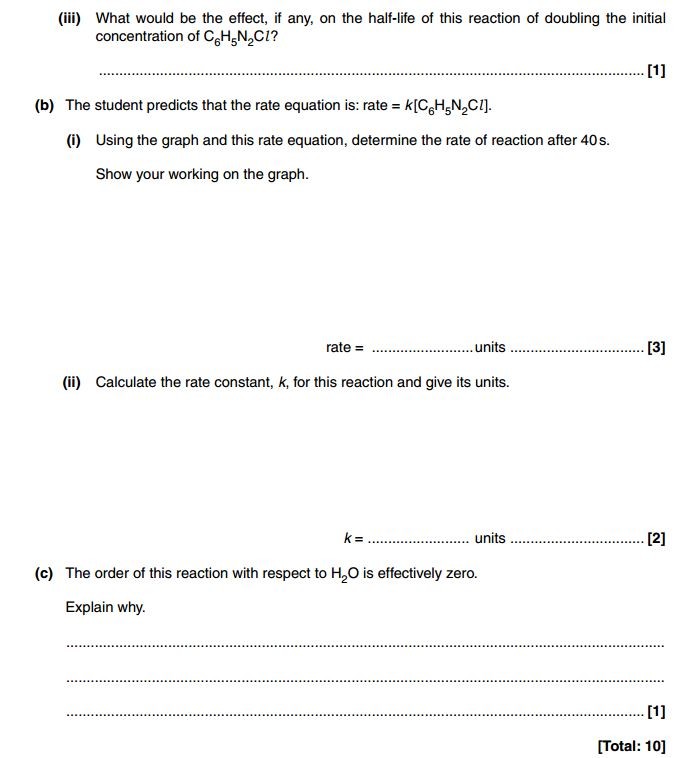
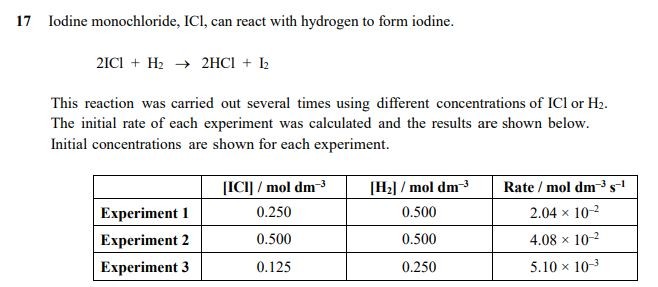
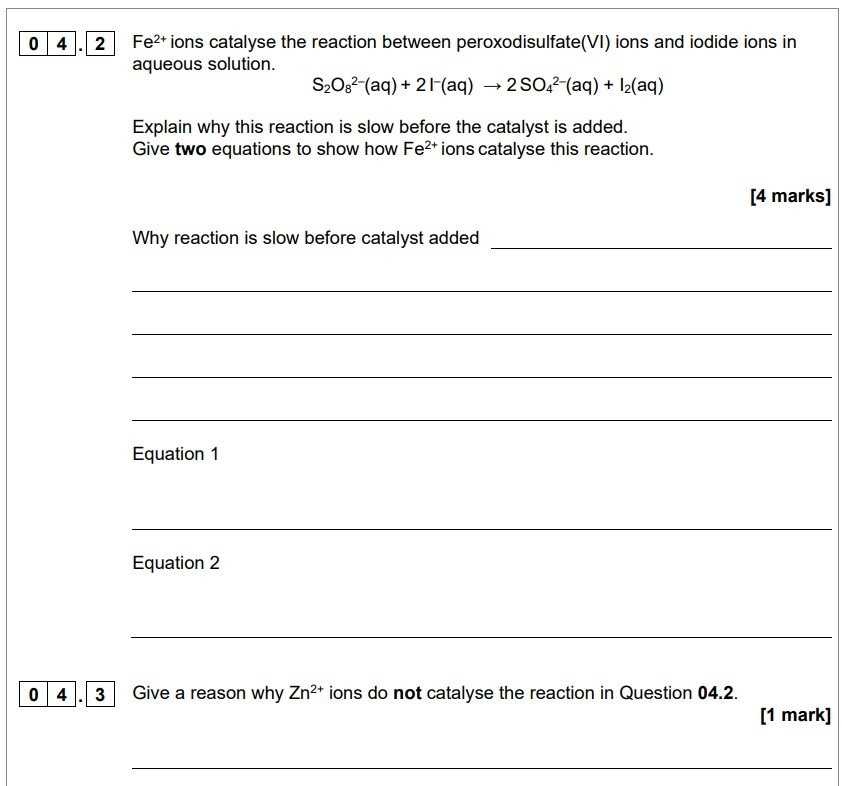
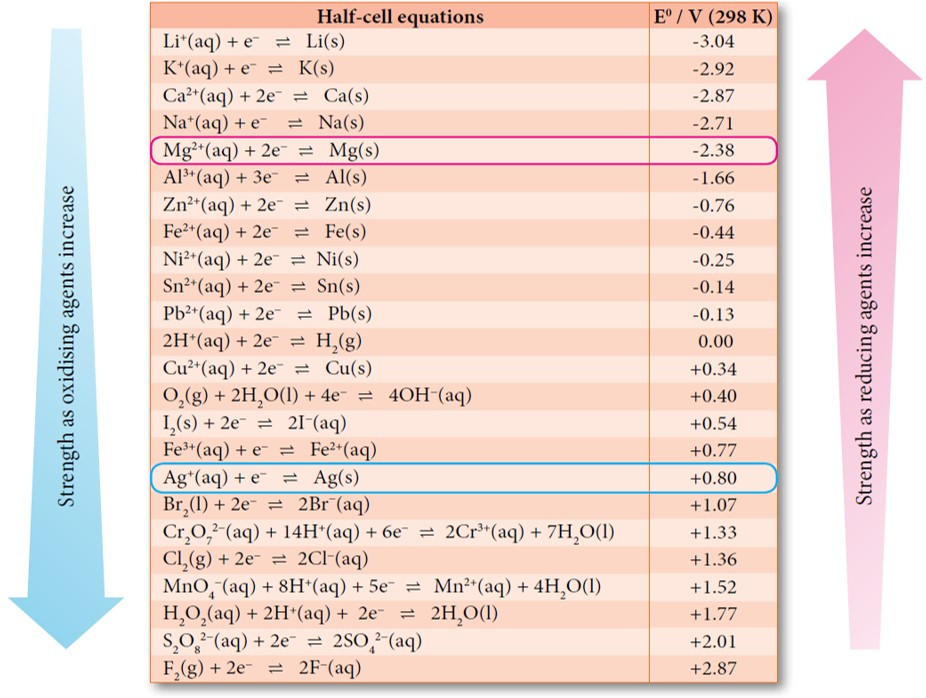
Exam Questions
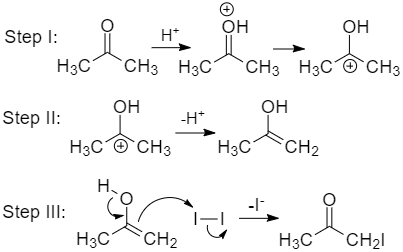
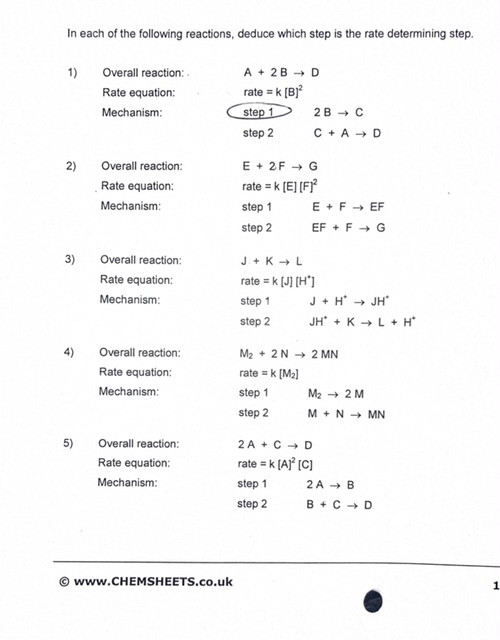
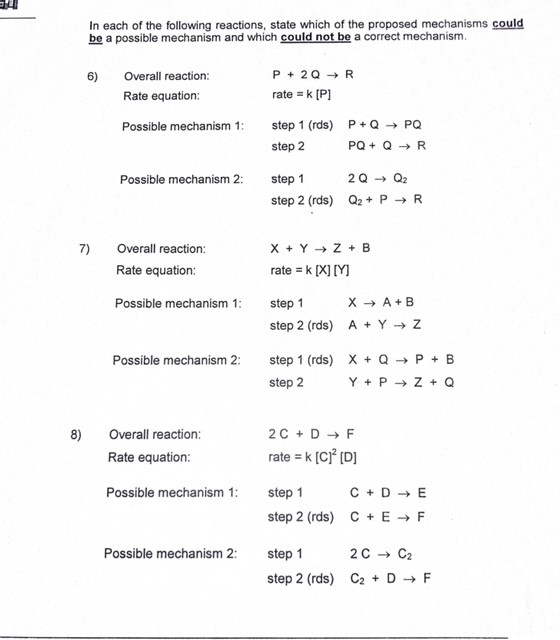
Two step mechanisms.
What rate equation is consistent with this mechanism?
How does substitution via this mechanism affect optical activity?
What are the implications of this mechanism for the rate equation?
How does substitution via this mechanism affect optical activity?

Entropy