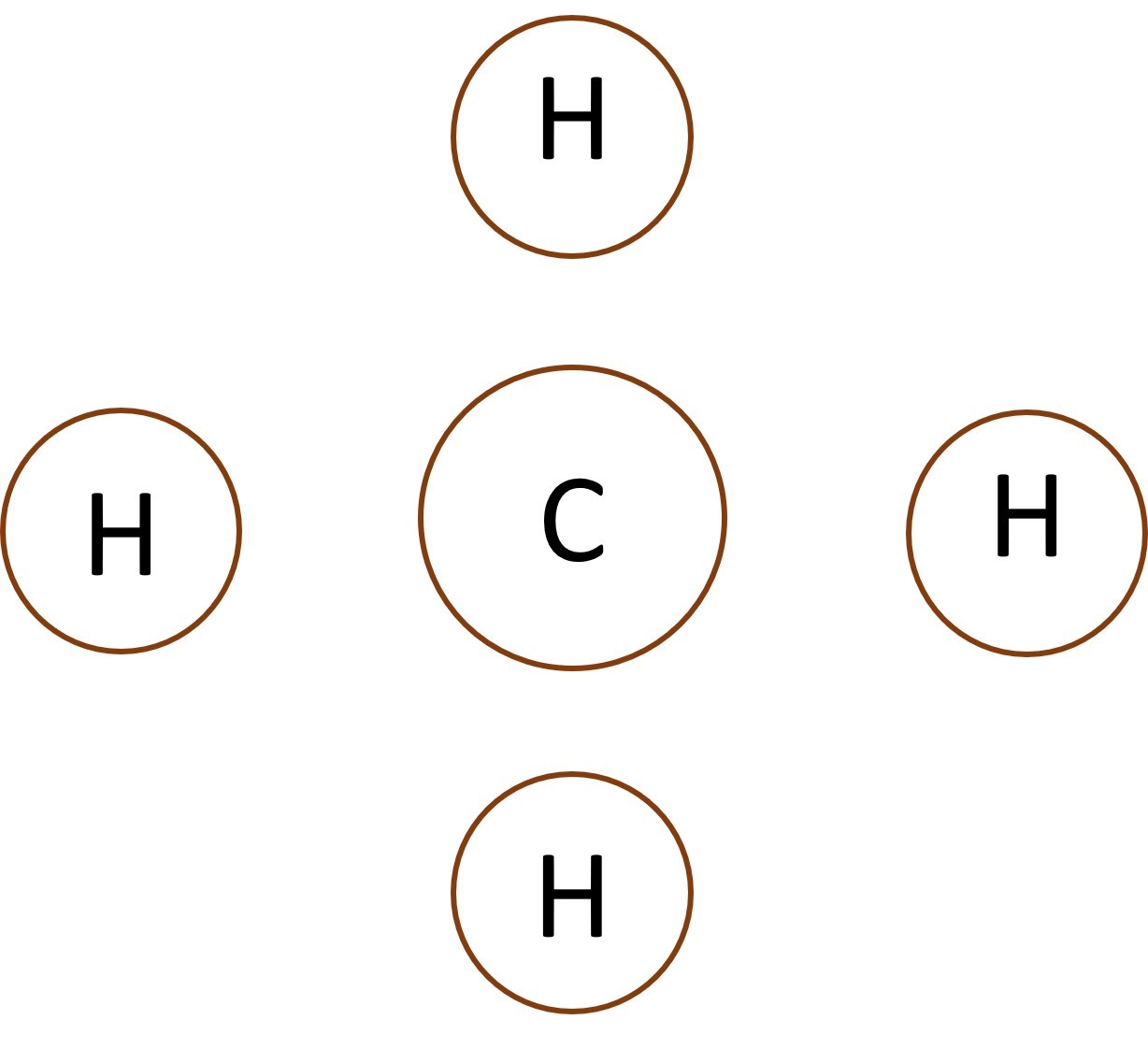
When non-metal atoms bond to other non-metal atoms they share electrons to form covalent bonds.
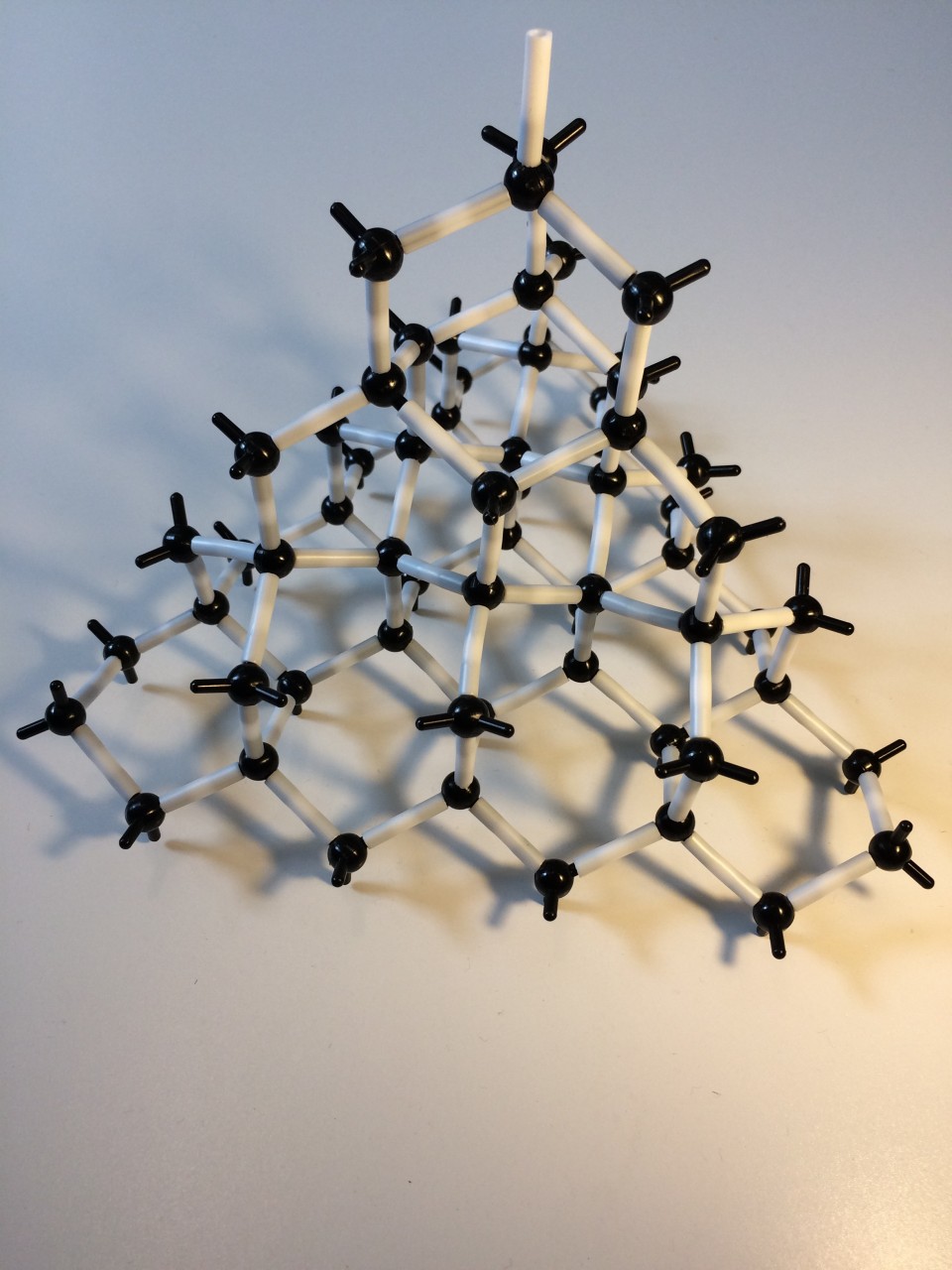
Diamond has a giant covalent structure .
in this topic, we have looked at the way in which ...