1.27 What is a mole? Students should: 1.27 know that the mole (mol) is the unit for the amount of a substance 1.28 understand how to carry...
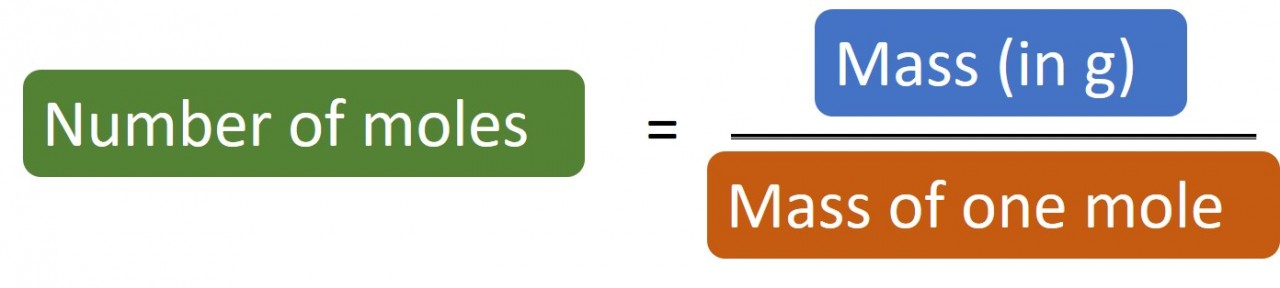
1.28 Calculating moles The following masses of elements all contain one mole of atoms: 12.0 g Carbon, 32.1 g Sulphur, 14 g Nitrogen,&n...
Relative masses allow chemists to "count out" atoms and molecules so they can ensure that the appropriate amounts of substances are reacted.
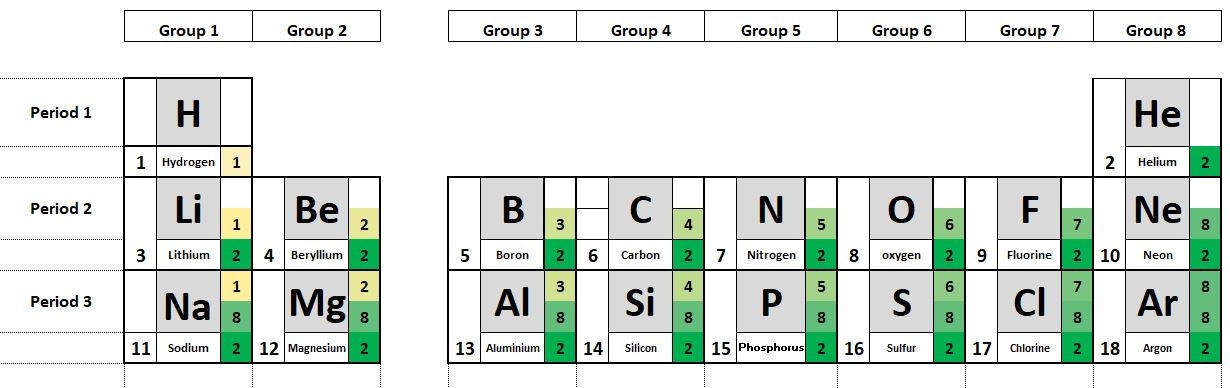
The elements in the periodic table are organised in order of increasing atomic number.
1.26 "Counting" by measuring mass Sometimes chemists need to "count" out a specific number of atoms, ions or molecules of a substance....
The elements in the periodic table are organised in order of increasing atomic number.
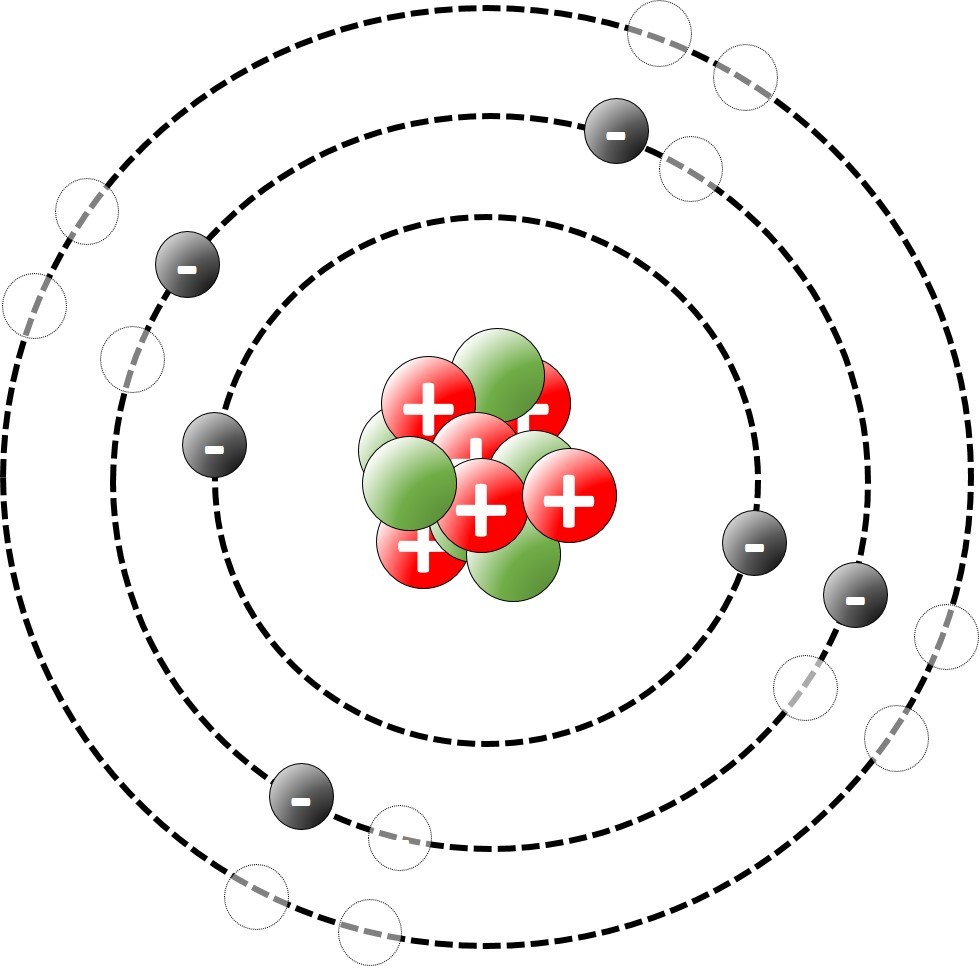
1.19 Deducing electron configurations Students should: 1.19 understand how to deduce the electronic configurations of the first 20 element...
1.20 - 1.22 Activity 2. Periodic variations Students should: 1.20 understand how to use electrical conductivity and the acid-base characte...
Mychem is a web-based resource dedicated to supporting the tutoring, teaching and learning of chemistry. It includes animations, i...
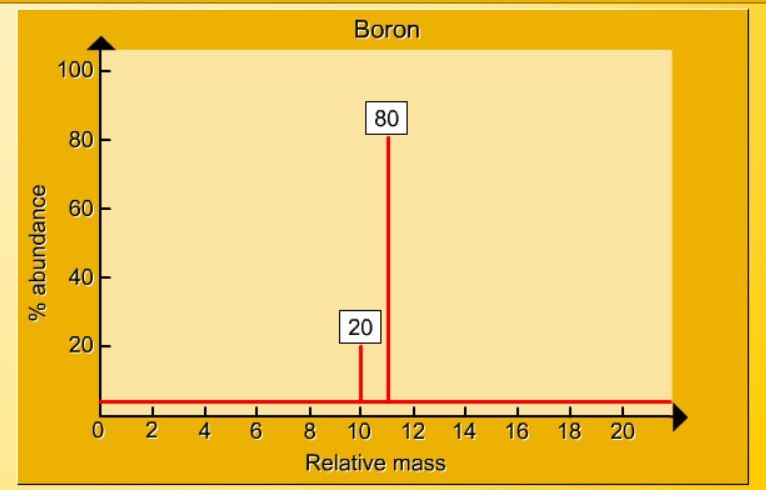
1.17 Activity 2. Calculating relative atomic mass. Students should: 1.17 be able to calculate the relative atomic mass of an element (Ar) ...