Chemical tests
Analytical chemistry involves the identification of chemical substances using a variety of methods.
Although modern technology has enabled chemists to analyse and identify a huge range of substances very quickly and accurately some simple test tube reactions can still be quicker and cheaper to perform.
| 2.44 describe tests for these gases: hydrogen, oxygen, carbon dioxide, ammonia, chlorine. |
| 2.45 describe how to carry out a flame test |
| 2.46 know the colours formed in flame tests for these cations: Li+ is red,Na+ is yellow, K+ is lilac, Ca2+ is orange-red, Cu2+ is blue-green. |
| 2.47 describe tests for these cations: NH4+ using sodium hydroxide solution and identifying the gas evolved, Cu2+, Fe2+ and Fe3+ using sodium hydroxide solution. |
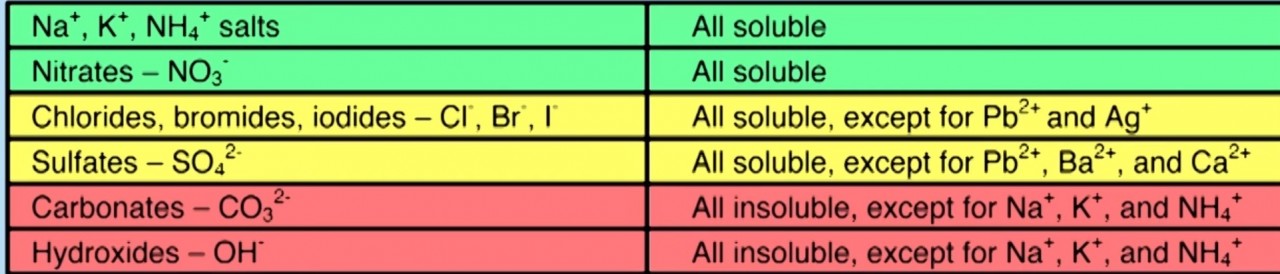
| 2.48 describe tests for these anions: Cl–, Br– and I– using acidified silver nitrate solution, SO42– using acidified barium chloride solution, CO32– using hydrochloric acid and identifying the gas evolved. |
| 2.49 describe a test for the presence of water using anhydrous copper(II) sulfate |
| 2.50 describe a physical test to show whether a sample of water is pure |
Enter your text here ...
Enter your text here ...

Testing hydrogen
Introduction Hydrogen is a flammable gas. It burn...
https://mychem.co.uk/index.php/igcse-chemistry/principles-of-chemistry/states-of-matter/testing-hydrogen

Testing for oxygen
Oxygen is a very reactive gas. It makes up a...
https://mychem.co.uk/index.php/igcse-chemistry/principles-of-chemistry/states-of-matter/test-for-oxygen
Stay Informed
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.