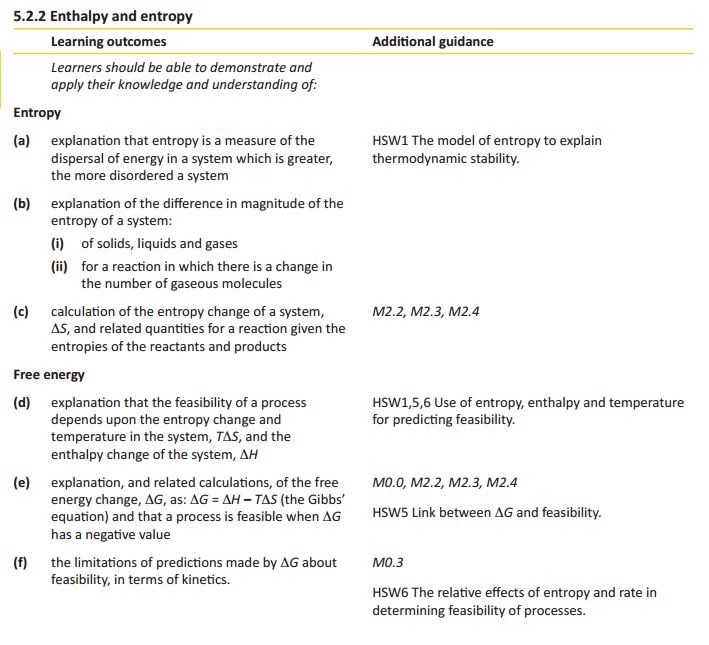
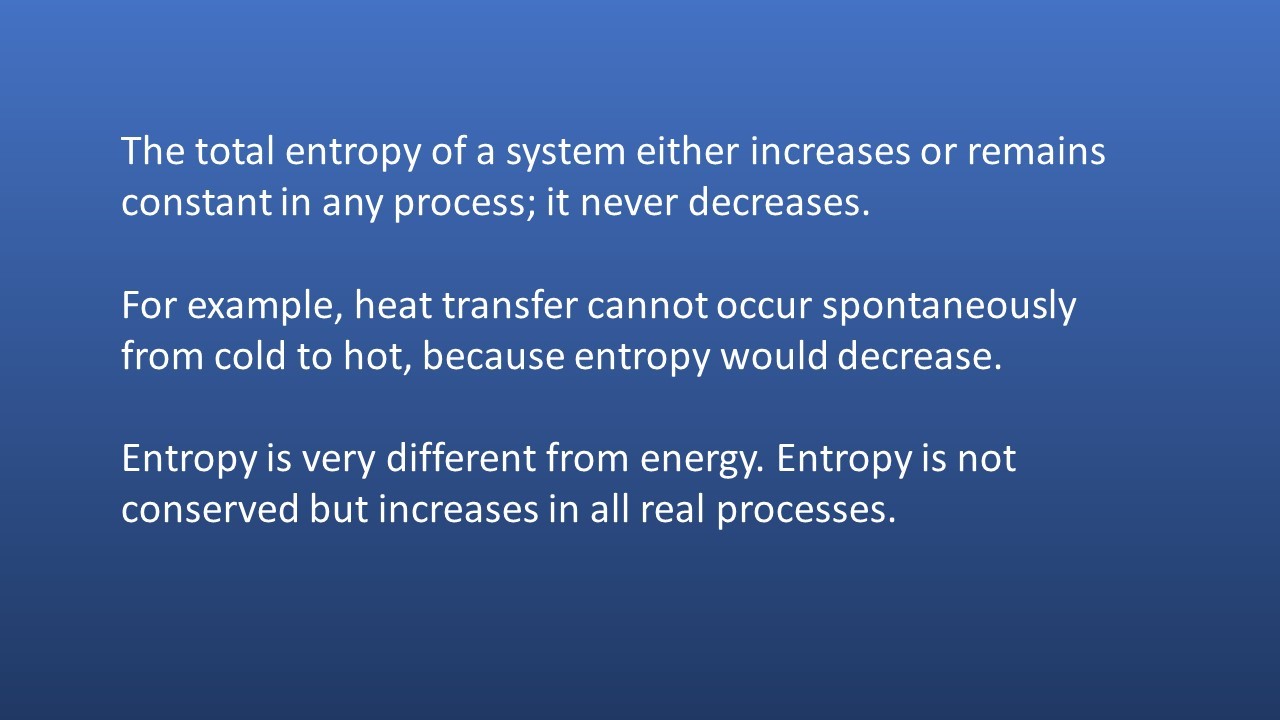
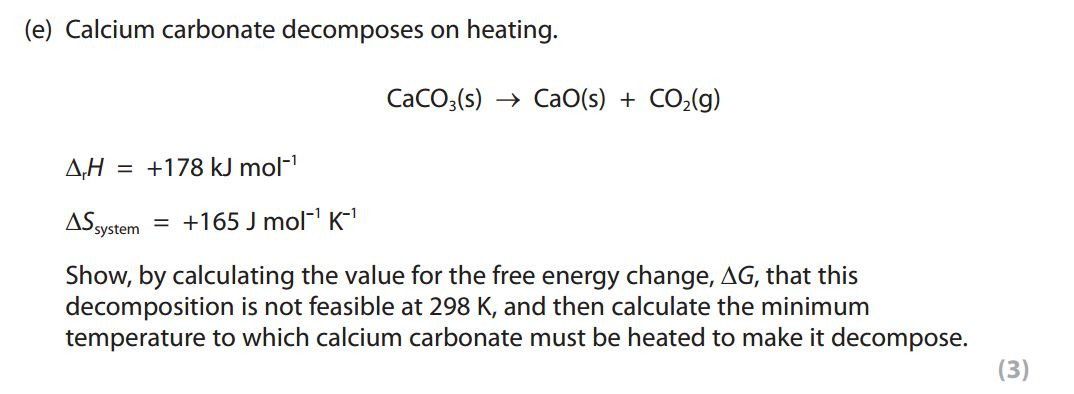
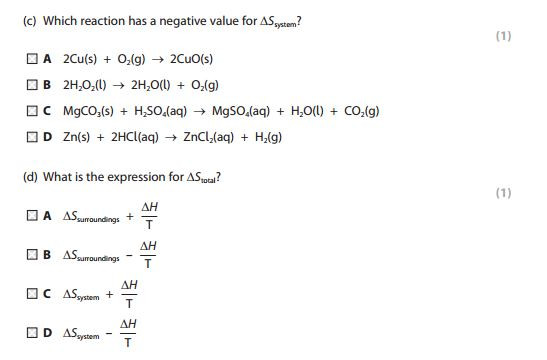Entropy
Exothermic reactions always lead to an increase in entropy of the surroundings. In an example like this - the burning of magnesium in oxygen - the entropy of the system decreases because a giant ionic lattice ( Magnesium Oxide) is formed from the magnesium metal and oxygen (gas).
Enter your text here ...
Stay Informed
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.