Batteries
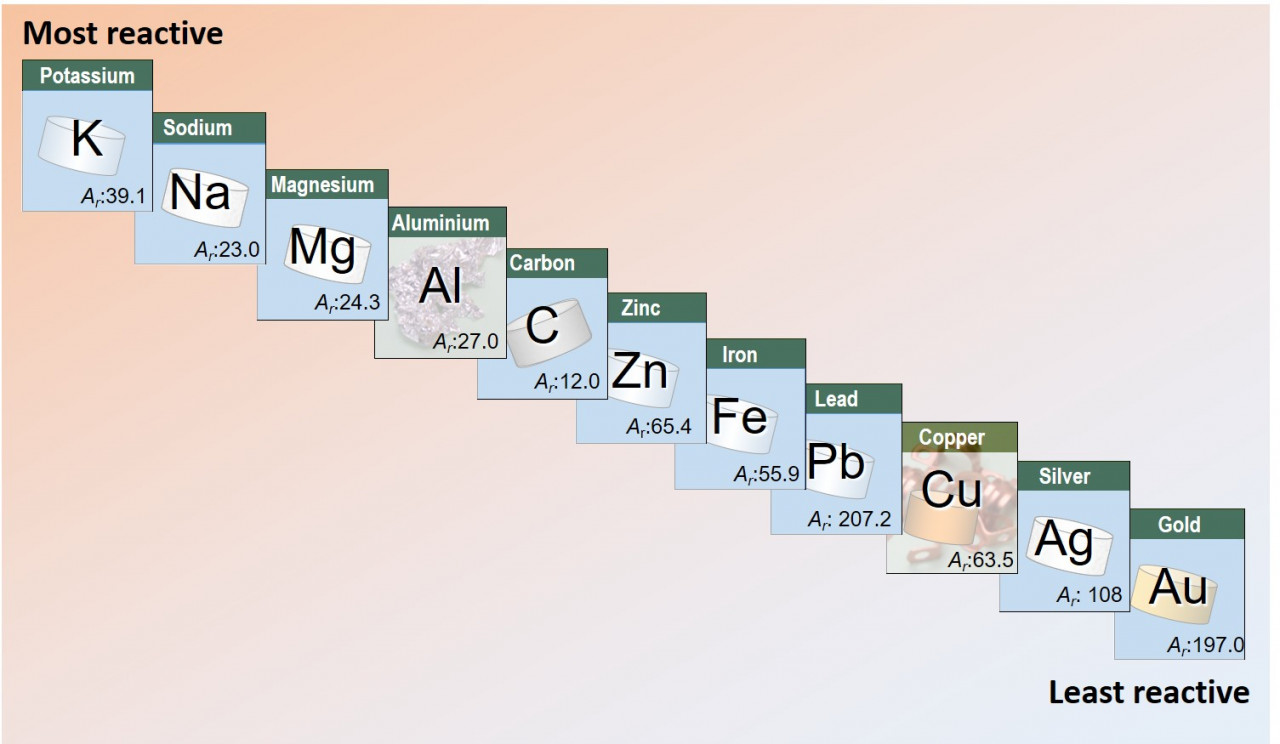
In the "furry worm" experiment the reactive magnesium metal reduces the less reactive copper.
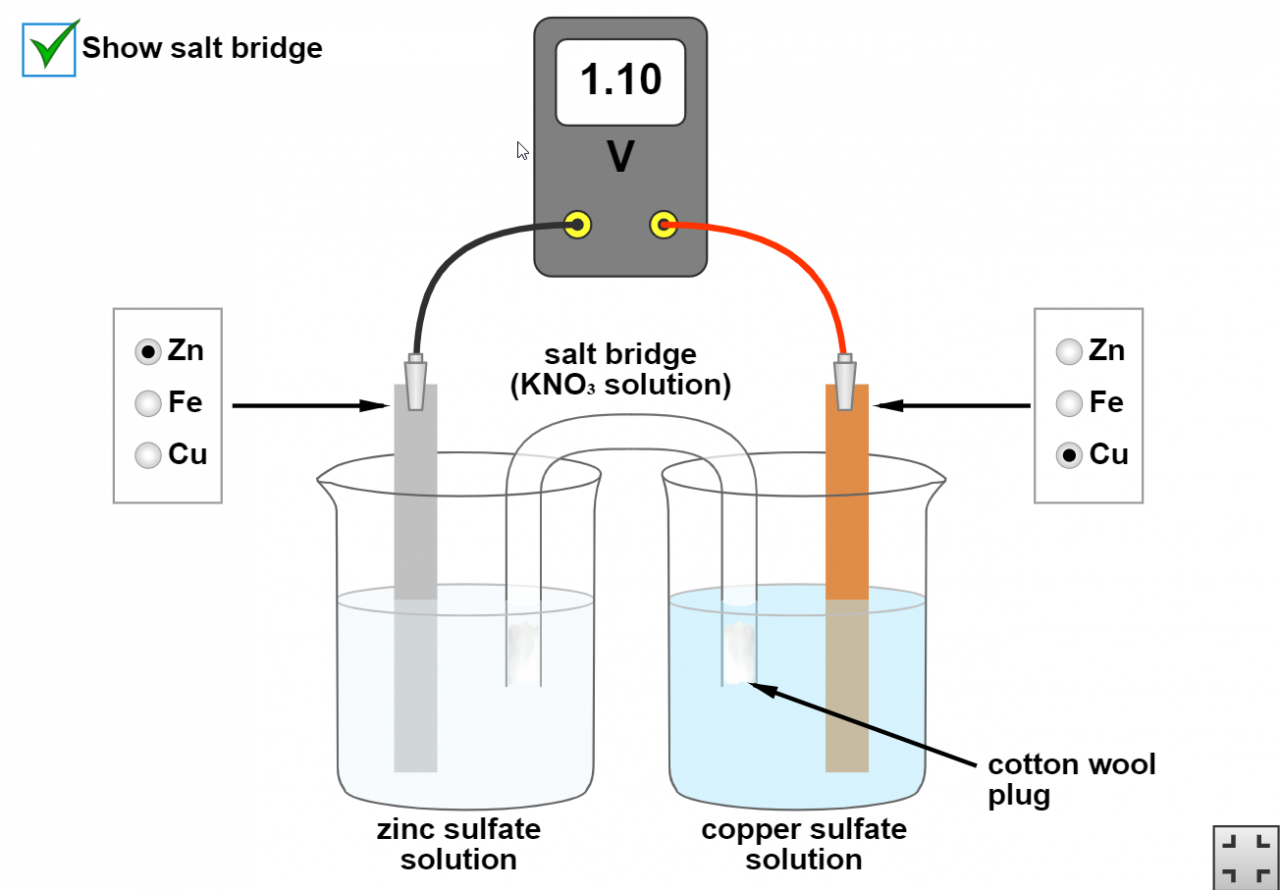
In this post we are going to investigate how metals can be used to make electric (voltaic) cells.
A reactive metal will transfer electrons to a less reactive metal. This can happen directly as in a displacement reaction or we can set things up so that the transfer of electrons happens via an electric circuit.
A more reactive metal will reduce a less reactive metal by giving electrons to the less reactive metal.
Stay Informed
When you subscribe to the blog, we will send you an e-mail when there are new updates on the site so you wouldn't miss them.